The overall dry AMD market size in the US is anticipated to increase, due to the uptake of recently approved therapies, the launch of emerging therapies such as Gildeuretinol (Alkeus Pharmaceuticals), Tinlarebant (Belite Bio), ANX007 (Annexon Biosciences), JNJ-1887 (Johnson & Johnson Innovative Medicine), AVD-104 (Aviceda Therapeutics), OpRegen (Lineage Cell Therapeutics and Roche), OCU410 (Ocugen), Elamipretide (Stealth BioTherapeutics), and others, an increase in diagnosis rate, and others.
New York, USA, Nov. 17, 2025 (GLOBE NEWSWIRE) — Dry Age-related Macular Degeneration Market Poised for Rapid Growth During the Forecast Period (2025–2034) Amid Expanding Treatment Landscape | DelveInsight
The overall dry AMD market size in the US is anticipated to increase, due to the uptake of recently approved therapies, the launch of emerging therapies such as Gildeuretinol (Alkeus Pharmaceuticals), Tinlarebant (Belite Bio), ANX007 (Annexon Biosciences), JNJ-1887 (Johnson & Johnson Innovative Medicine), AVD-104 (Aviceda Therapeutics), OpRegen (Lineage Cell Therapeutics and Roche), OCU410 (Ocugen), Elamipretide (Stealth BioTherapeutics), and others, an increase in diagnosis rate, and others.
DelveInsight’s Dry Age-related Macular Degeneration Market Insights report includes a comprehensive understanding of current treatment practices, emerging dry AMD drugs, market share of individual therapies, and current and forecasted dry AMD market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
Dry Age-related Macular Degeneration Market Summary
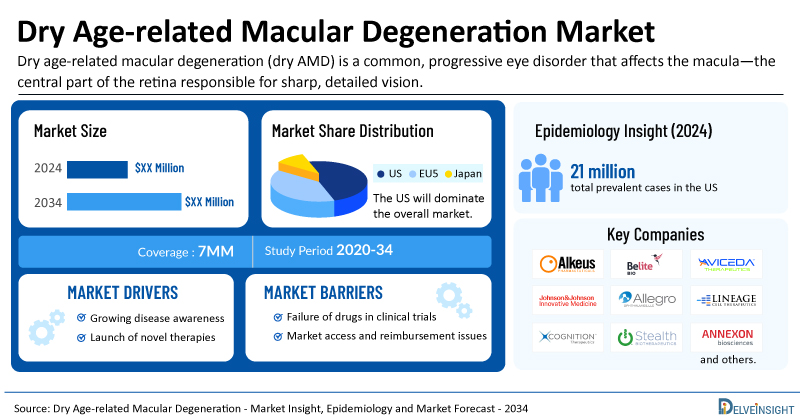
- The total dry AMD treatment market size is expected to grow positively by 2034 in the leading markets.
- The United States accounts for the largest market size of dry AMD, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- Among the 7MM, the US accounted for the highest prevalent cases of dry AMD in 2024, with around 21 million cases; these cases are expected to increase during the forecast period.
- Key dry AMD companies, including Alkeus Pharmaceuticals, Belite Bio, Aviceda Therapeutics, Johnson & Johnson Innovative Medicine, Allegro Ophthalmics, Lineage Cell Therapeutics (CellCure Neurosciences), Roche, Cognition Therapeutics, Stealth BioTherapeutics, Annexon Biosciences, and others, are actively working on innovative dry AMD drugs.
- Some of the key dry AMD therapies in clinical trials include Gildeuretinol (ALK-001), Tinlarebant (LBS-008), AVD-104, JNJ-1887, Luminate (risuteganib), OpRegen, CT1812, Elamipretide, ANX007, and others. These novel dry AMD therapies are anticipated to enter the dry AMD market in the forecast period and are expected to change the market.
Discover which dry AMD medications are expected to grab the market share @ Dry Age-related Macular Degeneration Market Report
Key Factors Driving the Growth of the Dry AMD Market
Rising Dry AMD Prevalence
Among the 7MM, the US accounted for the highest prevalent cases of dry AMD in 2023, with around 21 million cases; these cases are expected to increase by 2034.
Emerging Diagnostic Technologies in Ophthalmology
New diagnostic technologies such as spectral-domain OCT and fundus autofluorescence have emerged, giving ophthalmologists a better picture of the eye, which will aid in the confirmation of anatomic endpoints for geographic atrophy.
ANX007: A Major Step Forward for Patients Without Treatment Options
ANX007 is the first therapeutic candidate for the treatment of geographic atrophy to receive PRIME designation in the EU, which provides early and proactive support to developers of promising medicines that may offer a major therapeutic advantage over existing treatments or benefit patients without treatment options.
Emergence of Novel Dry AMD Drugs
Some of the drugs in the pipeline include Gildeuretinol (Alkeus Pharmaceuticals), Tinlarebant (Belite Bio), ANX007 (Annexon Biosciences), JNJ-1887 (Johnson & Johnson Innovative Medicine), AVD-104 (Aviceda Therapeutics), OpRegen (Lineage Cell Therapeutics and Roche), OCU410 (Ocugen), Elamipretide (Stealth BioTherapeutics), ZM-02 (Zhongmou Therapeutics), RTG-2023 (RetinalGeniX), and others.
Dry Age-related Macular Degeneration Market Analysis
The FDA has approved two therapies for dry age-related macular degeneration (AMD): IZERVAY (August 2023) and SYFOVRE (February 2023). Despite these approvals, dry AMD remains a vast untapped market opportunity, as it accounts for nearly 90% of all AMD cases.
In February 2025, the U.S. FDA expanded the label for Astellas’ IZERVAY, authorizing its use for longer-term treatment of geographic atrophy. This extension reinforces IZERVAY’s position as a leading and trusted option for thousands of patients since its 2023 launch.
Beyond approved drugs, management of dry AMD often involves nutritional supplements (such as the AREDS formulation) and lifestyle adjustments, which aim to slow disease progression and alleviate symptoms. However, these approaches offer variable results and fail to comprehensively target the underlying disease mechanisms.
Multiple biopharmaceutical companies are actively developing novel treatments for dry AMD, including Alkeus Pharmaceuticals (gildeuretinol), Allegro Ophthalmics (risuteganib), Belite Bio (tinlarebant), Johnson & Johnson Innovative Medicine (JNJ-1887), Annexon Biosciences (ANX007), Stealth BioTherapeutics (elamipretide), Lineage Cell Therapeutics (OpRegen), Zhongmou Therapeutics (ZM-02), and others.
ANX007, a pioneering therapy for geographic atrophy, has earned PRIME designation in the EU, marking a significant milestone in complement pathway inhibition. Meanwhile, Zhongmou Therapeutics’ ZM-02, a discovery-stage candidate, is being evaluated for dry AMD. Its mutation-independent mechanism could benefit a wide range of inherited and acquired retinal disorders characterized by photoreceptor degeneration, including AMD.
Additionally, RetinalGeniX’s RTG-2023, currently in pre-IND development for dry AMD, received a provisional patent in November 2023 and has successfully completed toxicity testing.
Learn more about the dry AMD treatment options @ Dry Age-related Macular Degeneration Treatment Market
Dry Age-related Macular Degeneration Competitive Landscape
Some of the dry AMD drugs in the clinical trials include Gildeuretinol (Alkeus Pharmaceuticals), Tinlarebant (Belite Bio), ANX007 (Annexon Biosciences), JNJ-1887 (Johnson & Johnson Innovative Medicine), AVD-104 (Aviceda Therapeutics), OpRegen (Lineage Cell Therapeutics and Roche), OCU410 (Ocugen), Elamipretide (Stealth BioTherapeutics), ZM-02 (Zhongmou Therapeutics), RTG-2023 (RetinalGeniX), and others.
Alkeus Pharmaceuticals’ Gildeuretinol is an investigational once-daily oral therapy currently being evaluated for geographic atrophy (GA). It is a chemically modified form of vitamin A designed to limit the buildup of toxic retinal by-products. Beyond GA, the drug is also being studied for Stargardt disease. Alkeus Pharmaceuticals recently completed its Phase II/III SAGA trial. In September 2024, the company reported that oral gildeuretinol acetate reduced GA lesion growth by 0.25 sq mm per year compared with placebo after 24 months. Further data released in May 2025 showed that patients receiving gildeuretinol experienced a slower decline in vision-related quality of life (VFQ-25) and functional reading independence (FRI) scores versus placebo over the same period.
Belite Bio’s Tinlarebant is another oral investigational therapy aimed at decreasing the buildup of toxic by-products in the retina that drive Stargardt disease type 1 (STGD1) and contribute to GA. These toxins form as a result of the vitamin A–dependent visual cycle. Belite Bio is conducting a global two-year Phase III PHOENIX study, a multicenter, randomized, double-masked, placebo-controlled trial evaluating tinlarebant’s safety and efficacy in GA. In November 2023, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) approved the PHOENIX trial. Belite Bio emphasizes that early intervention targeting non-inflammatory retinal damage could be key to slowing progression in STGD1 and GA.
Johnson & Johnson Innovative Medicine’s JNJ-81201887 (formerly AAVCAGsCD59) is a one-time gene augmentation therapy in development for geographic atrophy. The therapy is designed to elevate local expression of soluble CD59 (sCD59), potentially reducing membrane attack complex (MAC) formation and protecting retinal cells from degeneration. The U.S. FDA has granted it Fast Track designation, and the European Medicines Agency has recognized it as an Advanced Therapy Medicinal Product (ATMP). The Phase IIb PARASOL trial is now enrolling adults aged 60 and older with advanced dry AMD and GA to assess JNJ-1887’s therapeutic potential.
The anticipated launch of these emerging dry AMD therapies are poised to transform the dry AMD market landscape in the coming years. As these cutting-edge dry AMD therapies continue to mature and gain regulatory approval, they are expected to reshape the dry AMD market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for dry AMD, visit @ Dry Age-related Macular Degeneration Medication
Recent Developments in the Dry AMD Market
- In October 2025, PulseSight Therapeutics, focused on disruptive non-viral therapies with minimally invasive delivery, highlighted new data from a study by Inserm and Cochin Hospital, Paris, in collaboration with PulseSight scientists, supporting transferrin as a potential treatment for dry AMD.
- In February 2025, Cognition Therapeutics, reported a positive outcome of an analysis of masked data from the ongoing ‘MAGNIFY’ Phase II trial of zervimesine (CT1812) in adults with geographic atrophy secondary to dry AMD.
- In February 2025, Luxa Biotechnology announced that the US FDA has granted Regenerative Medicine Advanced Therapy (RMAT) designation to RPESC-RPE-4W transplantation for the treatment of patients with dry AMD.
- In February 2025, Ocugen announced that dosing is complete in the Phase II ArMaDa clinical trial for OCU410—a multifunctional modifier gene therapy for the treatment of geographic atrophy secondary to dry AMD.
- In February 2025, Annexon announced presentations on ANX007 in geographic atrophy at the Macula Society 48th Annual Meeting being held February 12-15 in Charlotte Harbor, Florida.
What is Dry Age-related Macular Degeneration?
Dry age-related macular degeneration (dry AMD) is a common, progressive eye disorder that affects the macula—the central part of the retina responsible for sharp, detailed vision. It occurs when the light-sensitive cells in the macula gradually deteriorate, resulting in blurred or reduced central vision, although peripheral vision usually remains intact. The condition is associated with the accumulation of tiny yellow deposits called drusen under the retina, which interfere with normal retinal function. Dry AMD typically progresses slowly over time and is more common than the “wet” form of AMD. While there is currently no cure, lifestyle modifications such as a healthy diet rich in antioxidants, smoking cessation, and specific vitamin supplements (AREDS2 formula) can help slow its progression and preserve vision.
Dry Age-related Macular Degeneration Epidemiology Segmentation
The dry AMD epidemiology section provides insights into the historical and current dry AMD patient pool and forecasted trends for the leading markets. The early and intermediate stages of AMD are major contributors among the stage-specific prevalent cases of AMD. In 2024, the late stage (nAMD and geographic atrophy) accounted for around ~1.7 million cases in the US.
The dry AMD market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- Total Prevalent Cases of AMD
- Stage-specific Prevalent Cases of AMD
- Total Prevalent Cases of Dry AMD
- Total Diagnosed Prevalent Cases of Dry AMD
- Total Prevalent Cases of Geographic Atrophy
- Age-specific Cases of Early and Intermediate AMD
- Age-specific Cases of Geographic Atrophy
- Geographic Atrophy Cases by Visual Impairment
Download the report to understand dry AMD management @ Dry Age-related Macular Degeneration Treatment Options
| Dry Age-related Macular Degeneration Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Dry Age-related Macular Degeneration Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Dry Age-related Macular Degeneration Epidemiology Segmentation | Total Prevalent Cases of AMD, Stage-specific Prevalent Cases of AMD, Total Prevalent Cases of Dry AMD, Total Diagnosed Prevalent Cases of Dry AMD, Total Prevalent Cases of Geographic Atrophy, Age-specific Cases of Early and Intermediate AMD, Age-specific Cases of Geographic Atrophy, and Geographic Atrophy Cases by Visual Impairment |
| Key Dry Age-related Macular Degeneration Companies | Alkeus Pharmaceuticals, Belite Bio, Aviceda Therapeutics, Johnson & Johnson Innovative Medicine, Allegro Ophthalmics, Lineage Cell Therapeutics (CellCure Neurosciences), Roche, Cognition Therapeutics, Stealth BioTherapeutics, Annexon Biosciences, Astellas Pharma, Iveric Bio, Apellis Pharmaceuticals, and others |
| Key Dry Age-related Macular Degeneration Therapies | Gildeuretinol (ALK-001), Tinlarebant (LBS-008), AVD-104, JNJ-1887, Luminate (risuteganib), OpRegen, CT1812, Elamipretide, ANX007, IZERVAY, SYFOVRE, and others |
Scope of the Dry Age-related Macular Degeneration Market Report
- Dry Age-related Macular Degeneration Therapeutic Assessment: Dry Age-related Macular Degeneration current marketed and emerging therapies
- Dry Age-related Macular Degeneration Market Dynamics: Conjoint Analysis of Emerging Dry Age-related Macular Degeneration Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Dry Age-related Macular Degeneration Market Unmet Needs, KOL’s views, Analyst’s views, Dry Age-related Macular Degeneration Market Access and Reimbursement
Discover more about dry AMD drugs in development @ Dry Age-related Macular Degeneration Clinical Trials
Table of Contents
| 1 | Dry AMD Market Key Insights |
| 2 | Dry AMD Market Report Introduction |
| 3 | Executive Summary of Dry AMD |
| 4 | Key Events |
| 5 | Epidemiology and Market Forecast Methodology |
| 6 | Dry AMD Market Overview at a Glance |
| 6.1 | Market Share by Therapies (%) Distribution of Dry AMD in 2024 in the 7MM |
| 6.2 | Market Share by Therapies (%) Distribution of Dry AMD in 2034 in the 7MM |
| 7 | Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | Dry AMD Diagnosis |
| 7.3 | Diagnostic Guidelines |
| 7.4 | Dry AMD Treatment |
| 7.5 | Dry AMD Treatment Guidelines |
| 8 | Epidemiology and Patient Population |
| 8.1 | Key Findings |
| 8.2 | Assumption and Rationale |
| 8.3 | Total Prevalent Cases of AMD in the 7MM |
| 8.4 | Total Prevalent Cases of Dry AMD in the 7MM |
| 8.5 | The United States |
| 8.5.1 | Total Prevalent Cases of AMD in the United States |
| 8.5.2 | Stage-specific Prevalent Cases of AMD in the United States |
| 8.5.3 | Total Prevalent Cases of Dry AMD in the United States |
| 8.5.4 | Total Diagnosed Prevalent Cases of Dry AMD in the United States |
| 8.5.5 | Total Prevalent Cases of Geographic Atrophy in the United States |
| 8.5.6 | Age-specific Cases of Early and Intermediate AMD in the United States |
| 8.5.7 | Age-specific Cases of Geographic Atrophy in the United States |
| 8.5.8 | Geographic Atrophy Cases by Visual Impairment in the US |
| 8.6 | EU4 and the UK |
| 8.7 | Japan |
| 9 | Dry AMD Patient Journey |
| 9.1 | Description |
| 10 | Marketed Dry AMD Drugs |
| 10.1 | Key Competitors |
| 10.2 | IZERVAY (avacincaptad pegol): Astellas Pharma/Iveric Bio |
| 10.2.1 | Product Description |
| 10.2.2 | Regulatory Milestones |
| 10.2.3 | Other Developmental Activities |
| 10.2.4 | Clinical Developmental Activities |
| 10.2.4.1 | Clinical Trial Information |
| 10.2.5 | Safety and Efficacy |
| 10.2.6 | Analysts’ Views |
| 10.3 | SYFOVRE (pegcetacoplan): Apellis Pharmaceuticals |
| 11 | Emerging Dry AMD Drugs |
| 11.1 | Key Competitors |
| 11.2 | Gildeuretinol (ALK-001): Alkeus Pharmaceuticals |
| 11.2.1 | Product Description |
| 11.2.2 | Other Developmental Activities |
| 11.2.3 | Clinical Developmental Activities |
| 11.2.3.1 | Clinical Trial Information |
| 11.2.4 | Safety and Efficacy |
| 11.2.5 | Analyst Views |
| 11.3 | Tinlarebant (LBS-008): Belite Bio |
| 11.4 | AVD-104: Aviceda Therapeutics |
| 11.5 | JNJ-1887: Johnson & Johnson Innovative Medicine |
| 11.6 | Luminate (risuteganib): Allegro Ophthalmics |
| 11.7 | OpRegen: Lineage Cell Therapeutics (CellCure Neurosciences) and Roche |
| 11.8 | CT1812: Cognition Therapeutics |
| 11.9 | Elamipretide: Stealth BioTherapeutics |
| 11.10 | ANX007: Annexon Biosciences |
| 12 | Dry AMD: Market Analysis |
| 12.1 | Key Findings |
| 12.2 | Dry AMD Market Outlook |
| 12.3 | Conjoint Analysis |
| 12.4 | Key Dry AMD Market Forecast Assumptions |
| 12.5 | Total Market Size of Dry AMD in the 7MM |
| 12.6 | United States Dry AMD Market Size |
| 12.6.1 | Total Market Size of Dry AMD in the United States |
| 12.6.2 | Market Size of Dry AMD by Therapies in the United States |
| 12.7 | EU4 and the UK Dry AMD Market Size |
| 12.8 | Japan Dry AMD Market Size |
| 13 | Dry AMD Market Unmet Needs |
| 14 | Dry AMD Market SWOT Analysis |
| 15 | KOL Views on Dry AMD |
| 16 | Dry AMD Market Access and Reimbursement |
| 16.1 | United States |
| 16.2 | EU4 and the UK |
| 16.3 | Japan |
| 16.4 | Summary and Comparison of Market Access and Pricing Policy Developments in 2025 |
| 16.5 | Market Access and Reimbursement of Dry AMD |
| 17 | Bibliography |
| 18 | Dry AMD Market Report Methodology |
Related Reports
Dry Age-related Macular Degeneration Clinical Trial Analysis
Dry Age-related Macular Degeneration Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key dry AMD companies, including Alkeus Pharmaceuticals, Dobecure, Belite Bio, Cognition Therapeutics, Aviceda Therapeutics, Stealth BioTherapeutics, Allergo Ophthalmics, Annexon, Inc., Johnson & Johnson, InflammX Therapeutics, Lineage Cell Therapeutics, Ionis Pharmaceuticals, Evergreen Therapeutics, Inc., Alexion, Luxa Biotechnology, Astellas Pharma, OliX Pharmaceuticals, Hoffmann-La Roche, Boehringer Ingelheim, Eyevensys, among others.
Age-related Macular Degeneration Market
Age-related Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key AMD companies, including Opthea Limited, Kodiak Sciences Inc., REGENXBIO, Alkahest Inc, Evergreen Therapeutics, Alkeus Pharmaceuticals, Ribomic USA Inc, Outlook Therapeutics, Inc., Unity Biotechnology, Inc, PanOptica, Inc., Clearside Biomedical, Alexion Pharmaceuticals, AstraZeneca, Alkeus Pharmaceuticals, Stealth BioTherapeutics, CellCure Neurosciences, Regenerative Patch Technologies, Allegro Ophthalmics, Annexon Biosciences, NGM Biopharmaceuticals, Ionis Pharmaceuticals, Apellis Pharmaceuticals, Iveric Bio, Gyroscope Therapeutics, Novartis, Luxa Biotechnology, Gemini Therapeutics, among others.
Geographic Atrophy Market
Geographic Atrophy Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key geographic atrophy companies, including Apellis Pharmaceuticals, Iveric Bio (formerly Ophthotech Corporation), Alkeus Pharmaceuticals, Hemera Biosciences, Allegro Ophthalmics, Stealth BioTherapeutics, Regenerative Patch Technologies, Novartis, Roche, Ionis Pharmaceuticals, CellCure Neurosciences (a subsidiary of Lineage Cell Therapeutics), among others.
Age-related Macular Degeneration Clinical Trial Analysis
Age-related Macular Degeneration Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key age-related macular degeneration companies including Regeneron Pharmaceuticals, Novartis, Roche, Opthea Limited, Kodiak Sciences Inc., REGENXBIO, Alkahest Inc, Graybug Vision, Ribomic USA Inc, Outlook Therapeutics, Inc., Unity Biotechnology, Inc, PanOptica, Inc., Clearside Biomedical, Alexion Pharmaceuticals, AstraZeneca, Evergreen Therapeutics, Alkeus Pharmaceuticals, Stealth BioTherapeutics, CellCure Neurosciences, Regenerative Patch Technologies, Allegro Ophthalmics, Annexon Biosciences, NGM Biopharmaceuticals, Ionis Pharmaceuticals, Apellis Pharmaceuticals, Iveric Bio, Gyroscope Therapeutics, Luxa Biotechnology, Gemini Therapeutics, among others.
Wet Age-related Macular Degeneration Market
Wet Age-related Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key wet AMD companies including EyePoint Pharmaceuticals, Inc., AbbVie, Caregen Co. Ltd., Exegenesis Bio, Shanghai Henlius Biotech, Skyline Therapeutics, 4D Molecular Therapeutics, Ocugenix Corporation, Adverum Biotechnologies, Inc., Ashvattha Therapeutics, Inc., AiViva BioPharma, Inc., Ocular Therapeutix, Inc., Clearside Biomedical, Inc., Hoffmann-La Roche, Kyowa Kirin, Inc., Opthea Limited, AffaMed Therapeutics Limited, EyeBiotech Ltd., Novartis, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com

Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaShorts takes no editorial responsibility for the same.
