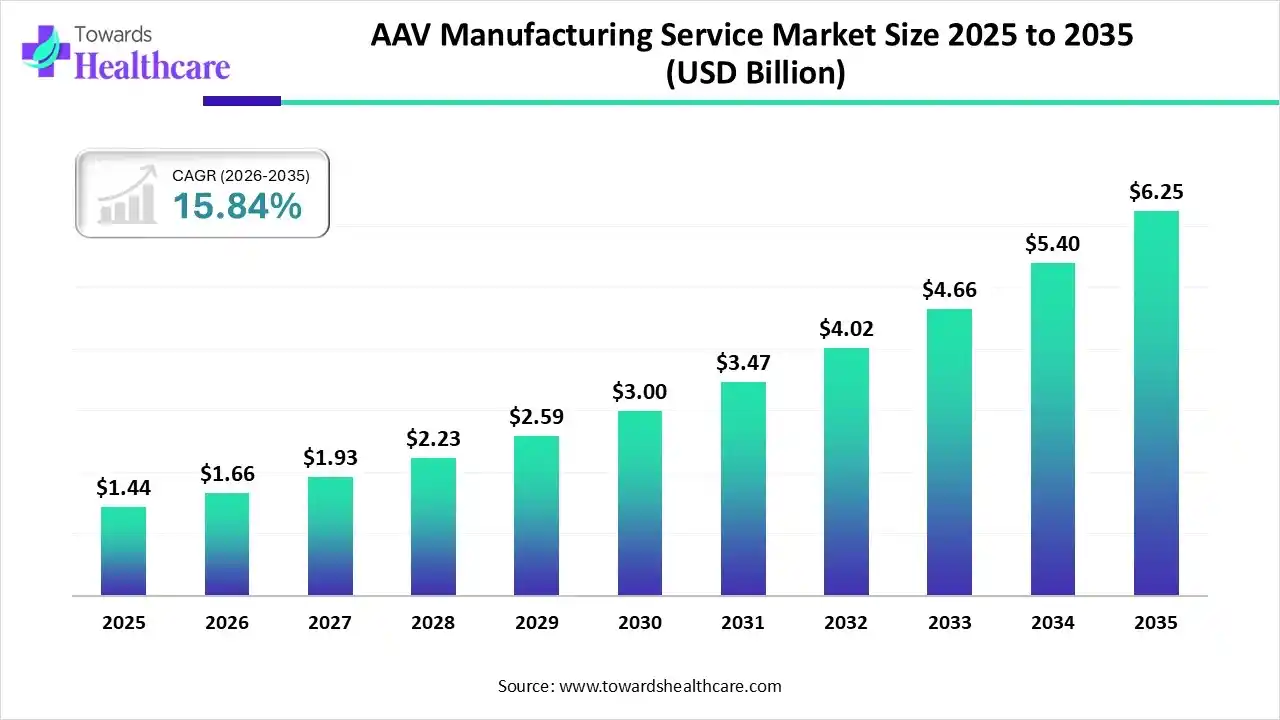
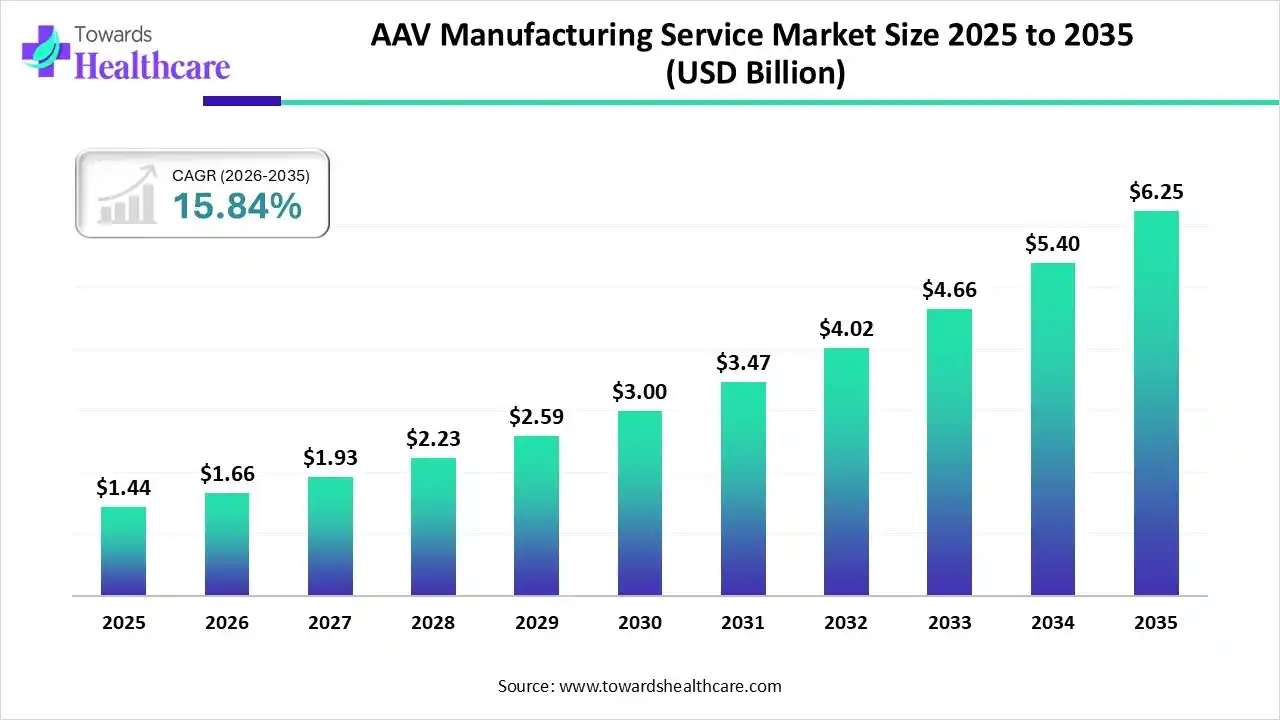
The global AAV manufacturing service market size is valued at USD 1.44 billion in 2025 and is predicted to hit around USD 6.25 billion by 2035, rising at a 15.84% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
Ottawa, Nov. 27, 2025 (GLOBE NEWSWIRE) — The global AAV manufacturing service market size is calculated at USD 1.66 billion in 2026 and is expected to reach around USD 6.25 billion by 2035, growing at a CAGR of 15.84% for the forecasted period, driven by the increasing diseases, R&D investments, and innovations.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/6345
Key Takeaways
- North America held a major revenue of approximately 40% share of the market in 2024.
- Asia-Pacific is expected to be the fastest-growing region in the AAV manufacturing service market between 2025-2034.
- By service type, the upstream manufacturing segment held a major revenue of approximately 45% share of the market in 2024.
- By service type, the downstream manufacturing segment in the market is expected to be the fastest-growing in the market during the forecast period.
- By vector type, the AAV9 segment held a major revenue of approximately 30% share of the market in 2024.
- By therapeutic area, the genetic/rare disorders segment held a major revenue of approximately 35% share of the market in 2024.
- By therapeutic area, the oncology segment is expected to be the fastest-growing in the market during the forecast period.
- By end-user, the pharmaceutical companies segment held a major revenue of approximately 45% share of the market in 2024.
- By end-user, the biotechnology companies segment is expected to be the fastest-growing in the market during the forecast period.
What is the AAV Manufacturing Service?
The AAV manufacturing services are driven by increasing demand for gene therapies. The AAV manufacturing services refer to the specialized services for producing adeno-associated virus vectors for gene therapy. These services are used for designing, purification, production, testing, and manufacturing of gene therapies, vaccines, and proteins.
What are the Major Growth Drivers in the AAV Manufacturing Service Market?
Growing genetic and rare diseases act as the major driver in the market. Therefore, due to their unmet need, the demand for AAV-based therapies is increasing, which is enhancing their development and innovations in pharmaceutical and biotech companies, increasing the vector production rates. Moreover, growing outsourcing trends, R&D investments, increasing clinical trials, and technological advances are some of the other market drivers.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What are the Key Drifts in the AAV Manufacturing Service Market?
The market has been expanding due to the growing collaborations to launch and enhance the use of various AAV manufacturing services.
- In November 2025, a non-exclusive worldwide license and collaboration between Solid Biosciences and Andelyn Biosciences was announced to provide AAV-SLB101 with the use of the AAV Curator® Platform for gene therapy clients.
- In August 2025, to advance the development and manufacturing of AVST-101, targeting X-linked retinoschisis (XLRS), a serious inherited retinal disease, a collaboration between Avista Therapeutics and Forge Biologics was announced.
- In July 2025, a collaboration between AAVnerGene Inc. and TFBS Bioscience Inc. was announced for enhancing the development and application of innovative adeno-associated virus (AAV) vector technologies for manufacturing. This collaboration will focus on enhancing the AAV tropism, manufacturing process, and production efficiency.
What is the Significant Challenge in the AAV Manufacturing Service Market?
Manufacturing complexity acts as the major limitation in the market. Due to the complex nature of AAV production, specialized expertise, staring quality control, and sophisticated equipment are required, which makes their manufacturing expensive. Moreover, limited capacity, payload limitations, and regulatory hurdles are other maker challenges.
Regional Analysis
Why did North America Dominate the AAV Manufacturing Service Market in 2024?
In 2024, North America captured the biggest revenue share of 40% in the market, due to the presence of robust industries focusing on the development of gene therapy. The advanced manufacturing infrastructure and R&D investments, and funding from various sources, increased the use of these services, driving the gene therapy innovations, as well as collaborations among the industries and institutions. This, in turn, also increased the clinical trials, which contributed to the market growth.
What Made Asia Pacific the Fastest Growing Region in the AAV Manufacturing Service Market in 2024?
Asia Pacific is expected to show the fastest growth in the market during the forecast period, due to increasing gene therapy research activities. At the same time, the expanding industries are also increasing their R&D, which are further being backed by investments, driving the demand for the AAV manufacturing services. Moreover, the growing disease burden and CDMOs are also leveraging these services at affordable prices. Thus, all these advancements with growing collaborations are promoting the market growth.
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
Segmental Insights
By service type analysis
Why Did the Upstream Manufacturing Segment Dominate in the AAV Manufacturing Service Market in 2024?
By service type, the upstream manufacturing segment led the market with approximately 45% share in 2024, due to the rapid expansion of gene therapies. At the same time, the adoption of advanced technologies also improved their workflow, driving their demand. Moreover, it also provided consistent and high revenue opportunities.
By service type, the downstream manufacturing segment is expected to show rapid growth during the forecast period, due to growing demand for high-purity products. This, in turn, is increasing their innovations to ensure their safety and scalability. Moreover, they are being used in the production of higher yields.
By vector type analysis
Which Vector Type Segment Held the Dominating Share of the AAV Manufacturing Service Market in 2024?
By vector type, the AAV9 segment held the dominating share of approximately 30% in the market in 2024, as it provides high transfection efficiency. They also demonstrated the ability to cross the blood-brain barrier, which increased their use in the development of neurological gene therapies. Furthermore, their enhanced safety profile increased their use in various applications.
By therapeutic area analysis
What Made Genetic/Rare Disorders the Dominant Segment in the AAV Manufacturing Service Market in 2024?
By therapeutic area, the genetic/rare disorders segment led the market with approximately 35% share in 2024, due to its ability to correct the gene defects. Additionally, their clinical studies also increased their adoption. Therefore, they are being preferred as a one-time treatment option, which was backed by investments.
By therapeutic area, the oncology segment is expected to show the highest growth during the forecast period, due to its growing incidence. At the same time, the growing R&D and expanding pipelines are also increasing the demand for these services. Additionally, the growing development of targeted therapies, supported by investments, is also increasing their innovations.
By end-user analysis
How the Pharmaceutical Companies Segment Dominated the AAV Manufacturing Service Market in 2024?
By end-user, the pharmaceutical companies segment held the largest share of approximately 45% of the market in 2024, due to the strong clinical pipeline. The growth in the R&D investments also increased their demand. Furthermore, the rise in outsourcing trends also contributed to their increased use.
By end-user, the biotechnology companies segment is expected to show rapid growth during the forecast period, due to growing startups. This is increasing the R&D, which is increasing the demand for these services. Additionally, the growing disease and funding are also encouraging the use of these services.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Recent Developments in the AAV Manufacturing Service Market
- In October 2025, AAV Edge, a stable producer system to tackle the scalability and cost challenges of adeno-associated virus (AAV) gene therapy manufacturing, was launched by Asimov.
- In August 2025, a cGMP Adeno-Associated Virus (AAV) manufacturing service was launched by ProBio at its 128,000 sq. ft. facility in Hopewell, New Jersey.
Also Read more from AAV Market:
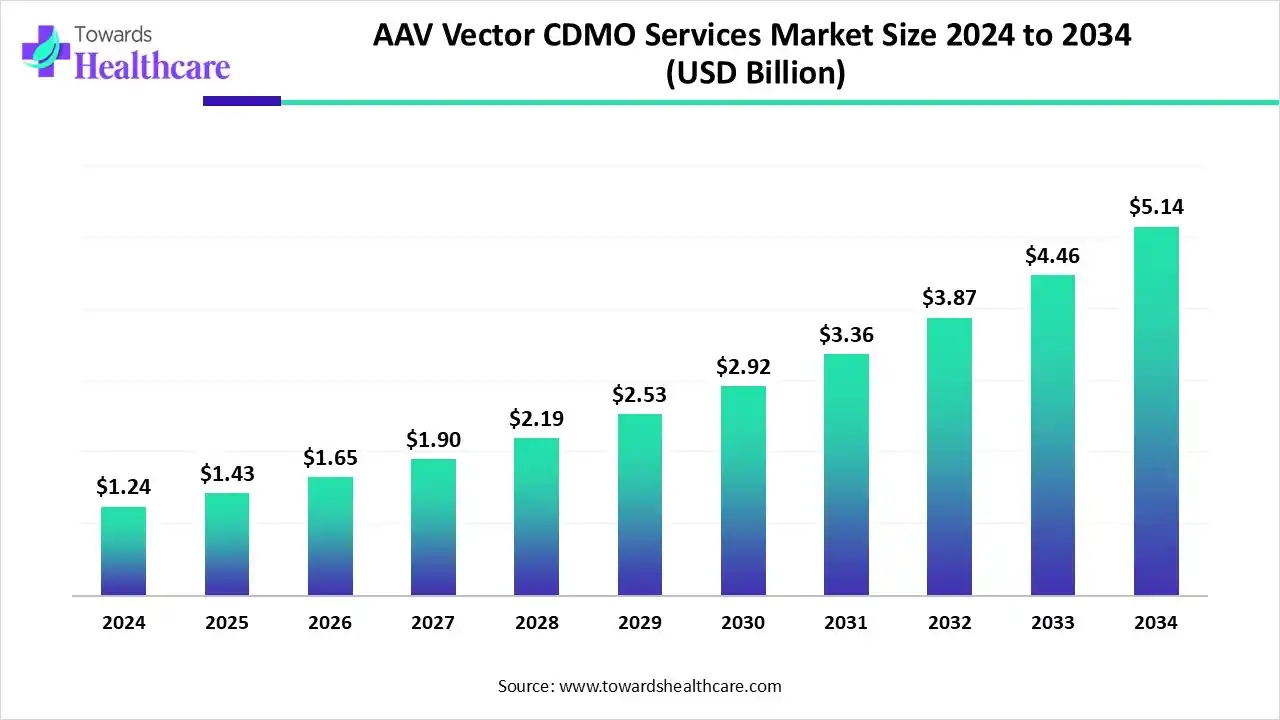
AAV Vector CDMO Services Market
The global AAV vector CDMO services market size is calculated at USD 1.24 billion in 2024, grew to USD 1.43 billion in 2025, and is projected to reach around USD 5.14 billion by 2034. The market is expanding at a CAGR of 15.24% between 2025 and 2034.
AAV Gene Therapy Market
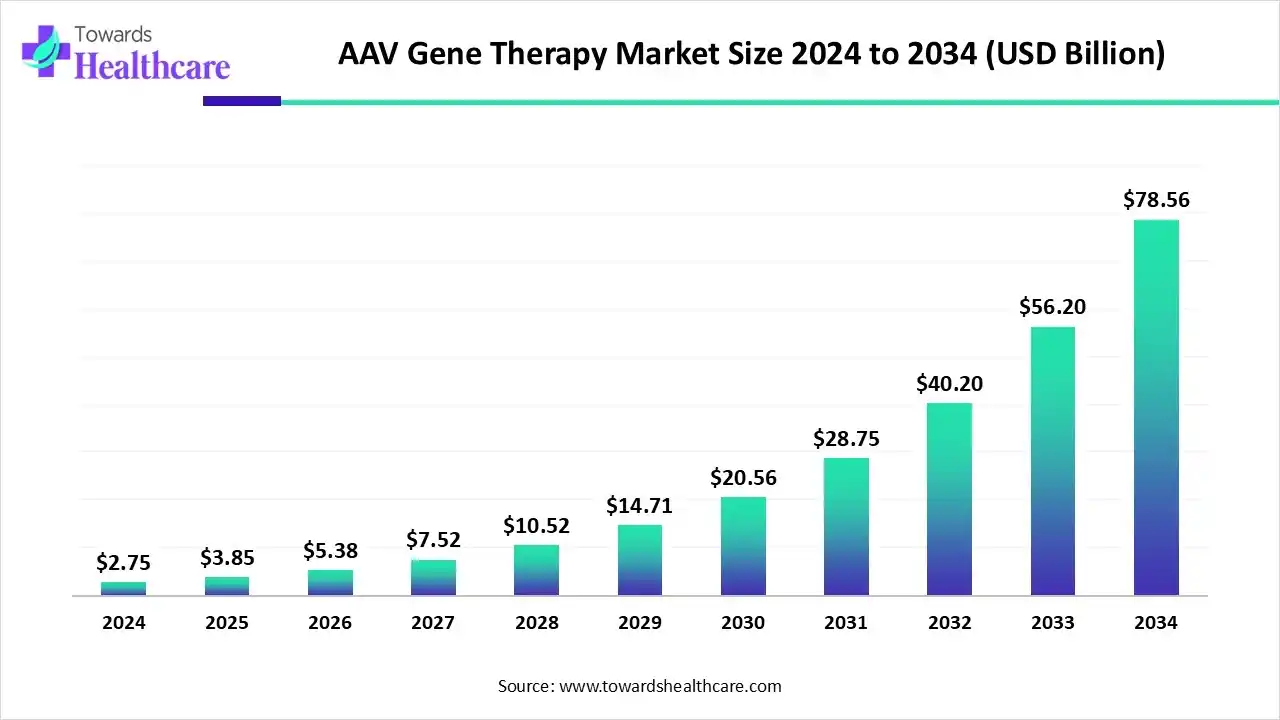
The AAV gene therapy market size was estimated at US$ 2.75 billion in 2024, projected to increase to US$ 3.85 billion in 2025 and reach US$ 78.56 billion by 2034, showing a healthy CAGR of 40.1% across the forecast years.
Multiple AAV Serotypes Market
The multiple AAV serotypes market is experiencing significant expansion, with projections indicating a revenue increase reaching several hundred million dollars by the end of the forecast period, spanning 2025 to 2034. This growth is driven by emerging trends and strong demand across key sectors.
AAV Manufacturing Service Market Key Players List
- WuXi AppTec
- Lonza Group
- Curia (formerly AMRI)
- Catalent Inc.
- AskBio (Asklepios BioPharmaceuticals)
- Vigene Biosciences
- REGENXBIO
- Brammer Bio (Thermo Fisher)
- GenScript Biotech
- Oxford Biomedica
- MeiraGTx
- Paragon Bioservices
- BioVectra
- Astellas Gene Therapy
- Fujifilm Diosynth Biotechnologies
- Sarepta Therapeutics
- AGC Biologics
- Spark Therapeutics
- CleanCap Technologies
- Cobra Biologics
Segments Covered in The Report
By Service Type
- Upstream Manufacturing
- Cell Line Development
- Plasmid Production
- Viral Vector Production/Transfection
- Downstream Manufacturing
- Purification & Concentration
- Formulation & Fill-Finish
- Analytical & Quality Control Services
- Titer & Potency Testing
- Impurity Testing
- Sterility & Safety Testing
- Others
By Vector Type
- AAV2
- AAV5
- AAV8
- AAV9
- Others
By Therapeutic Area
- Genetic/Rare Disorders
- Oncology
- CNS/Neurology
- Ophthalmology
- Cardiovascular Diseases
- Others
By End-User
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic & Research Institutes
By Region
North America
- U.S.
- Canada
- Mexico
- Rest of North America
South America
- Brazil
- Argentina
- Rest of South America
Europe
- Western Europe
- Germany
- Italy
- France
- Netherlands
- Spain
- Portugal
- Belgium
- Ireland
- UK
- Iceland
- Switzerland
- Poland
- Rest of Western Europe
- Eastern Europe
- Austria
- Russia & Belarus
- Türkiye
- Albania
- Rest of Eastern Europe
Asia Pacific
- China
- Taiwan
- India
- Japan
- Australia and New Zealand,
- ASEAN Countries (Singapore, Malaysia)
- South Korea
- Rest of APAC
MEA
- GCC Countries
- Saudi Arabia
- United Arab Emirates (UAE)
- Qatar
- Kuwait
- Oman
- Bahrain
- South Africa
- Egypt
- Rest of MEA
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/6345
Access our exclusive, data-rich dashboard dedicated to the healthcare market – built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaShorts takes no editorial responsibility for the same.