Revolutionary technologies like pulsed field ablation and structural heart therapies are driving rapid market expansion. Regulatory acceleration, workforce automation, and integrated patient monitoring are transforming clinical workflows, ensuring sustained industry growth and global adoption.
Chicago, Jan. 16, 2026 (GLOBE NEWSWIRE) — The global cardiovascular devices market size was valued at USD 74.58 billion in 2025 and is projected to hit the market valuation of USD 157.32 billion by 2035 at a CAGR of 7.70% during the forecast period 2026–2035.
Rising disease prevalence guarantees long-term demand for the cardiovascular devices market. It has been estimated that nearly 12.1 million people in the US are projected to be diagnosed with Atrial Fibrillation by 2030. Moreover, 127.9 million US adults had some form of cardiovascular disease in 2024. Mortality figures remain high. Cardiovascular disease accounted for 19.91 million global deaths in the latest 2024 report. As per WHO findings, stroke caused 162,890 deaths in the US. Total US cardiovascular deaths hit 931,578. The age-adjusted death rate rose to 233.3 per 100,000. China accounted for 22.26% of the global burden.
Request Sample Pages: https://www.astuteanalytica.com/request-sample/cardiovascular-devices-market
New technologies in the cardiovascular diseases are combating these statistics with superior efficiency. Medtronic’s PulseSelect demonstrated an average skin-to-skin procedure time of just 36 minutes in 2024. Remapping showed 98% of pulmonary veins remained isolated. Outcomes are improving drastically. TriClip patients were 28% less likely to be hospitalized for heart failure. 90% of TriClip patients saw regurgitation reduction. Varipulse achieved an 80% recurrence-free rate. Moreover, Boston Scientific’s Farapulse procedures finished in under 90 minutes in 90% of cases. Thermal ablation only achieved this speed in 60% of cases. The cardiovascular devices market is delivering measurable value.
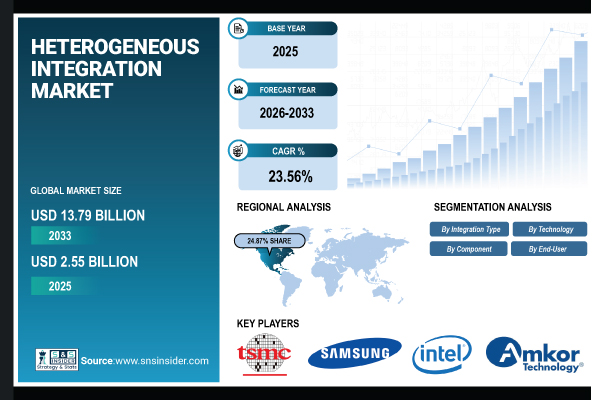
Key Findings
- By Device Type: The therapeutics & surgical devices category held a commanding position, accounting for a 77.5% revenue share in 2025.
- By Application: The coronary artery disease sector maintained supremacy within the global cardiovascular devices market in 2025.
- By End User: Hospitals represent the primary purchaser base for cardiovascular devices in the current market landscape.
- By Region: North America secured the largest geographical portion, contributing 45.68% of the revenue share in 2025.
Therapeutics Sector Captures 77.5% Share via High Value Implant Adoption
The therapeutics and surgical devices category secured a commanding 77.5% revenue share in 2025, driven largely by the high average selling price (ASP) of advanced implantable technologies. Unlike commoditized diagnostic consumables, therapeutic solutions such as cardiac rhythm management (CRM) systems and structural heart implants command premium pricing due to their life-saving complexity. Manufacturers within the Cardiovascular devices market have successfully integrated connectivity and remote monitoring into these implants, creating value-added ecosystems that justify higher costs. Such innovation ensures that therapeutic devices remain the financial backbone of the industry.
Revenue dominance in the cardiovascular devices market is further bolstered by the recurring nature of device replacements and upgrades. Defibrillators and pacemakers have defined battery lifecycles, guaranteeing a steady stream of replacement procedures that sustain market numbers. Furthermore, the expansion of indications for devices like Left Atrial Appendage (LAA) closure systems has opened entirely new revenue channels. Physicians are increasingly opting for these device-based therapies over long-term pharmaceutical management for stroke prevention, shifting healthcare spend directly toward device manufacturers. Consequently, the therapeutics segment captures the vast majority of healthcare capital allocated to cardiac care.
Coronary Artery Disease Leads Cardiovascular Devices Market with Unrivaled Procedure Volumes
Coronary Artery Disease (CAD) applications maintained their supremacy in the 2025 landscape, largely due to the acute and often non-elective nature of the condition. Acute Coronary Syndromes (ACS) demand immediate intervention, ensuring a recession-proof demand for coronary stents and balloons. This specific segment of the Cardiovascular devices market benefits from a “treat-and-release” efficiency model where percutaneous coronary intervention (PCI) allows for rapid patient turnover. High procedural throughput directly translates into massive volume consumption of interventional hardware, securing the sector’s top rank.
Growth is also underpinned by the increasing complexity of cases now being treated via minimally invasive techniques. Interventional cardiologists are tackling Chronic Total Occlusions (CTO) and calcified lesions that were previously referred for open surgery. Advanced tools such as atherectomy devices and specialized guide wires enable these high-complexity procedures, driving incremental revenue growth. Moreover, the integration of intravascular imaging (IVUS and OCT) during stent placement has become a best practice. Such synergy between imaging and therapy creates a multiplier effect on revenue, as optimal stent sizing requires the use of multiple high-cost ancillary devices during a single case.
Hospitals Dominate Purchasing Landscape Securing Major Market Revenue Share
Hospitals firmly represent the primary purchaser base for cardiovascular devices market, driven by the consolidation of healthcare delivery into large-scale Centers of Excellence. These institutions are the sole providers equipped with Hybrid Operating Rooms necessary for advanced structural heart procedures like TAVR and mitral valve repair. The market relies heavily on these high-acuity settings, as they possess the capital budgets required to purchase multimillion-dollar robotic navigation systems and heavy imaging equipment. Centralized procurement through hospital networks ensures that these facilities dictate the bulk of industry order volumes.
Supply chain dynamics further cement hospital dominance. Large hospital systems leverage Group Purchasing Organizations (GPOs) to negotiate bulk contracts for high-volume items like catheters and guidewires. While this exerts pricing pressure, it simultaneously guarantees massive volume commitments that other end-users cannot match. Additionally, the management of critical postoperative care requires Intensive Care Unit (ICU) support found almost exclusively in hospitals. Complex device implantations mandate this level of backup, ensuring that the most expensive and advanced technologies flow primarily through hospital inventory channels rather than ambulatory settings.
Pulsed Field Ablation Technology Driving Unprecedented Revenue and Safety Milestones
The rapid commercialization of Pulsed Field Ablation (PFA) is fundamentally altering the competitive landscape of the Cardiovascular devices market. Boston Scientific generated over USD 1 billion in revenue from its Farapulse system within the first full year of commercial sales in 2024, a milestone that underscores the industry’s decisive shift away from thermal ablation. Such immediate financial success is supported by high clinical throughput, with weekly procedure rates averaging 16,200 cases throughout 2024. Medtronic also solidified this trend, reporting over 10,000 procedures with its PulseSelect system by October 2024. These volumes indicate that electrophysiology labs are aggressively replacing legacy cryoablation and radiofrequency tools with PFA systems to increase efficiency.
Safety profiles are acting as the primary catalyst for this rapid equipment upgrade cycle. The MANIFEST-17K study, which analyzed 17,642 patients in 2024, reported a 0% rate of esophageal damage, a complication that historically plagued thermal technologies. Furthermore, the same study revealed a pericardial tamponade rate of just 0.36%. Medtronic’s PulseSelect reinforced these findings by achieving 100% acute pulmonary vein isolation in real-world evaluations. Hospitals are now prioritizing PFA procurement to mitigate liability and improve patient turnover, driving a massive capital equipment replacement wave across the sector.
Structural Heart Interventions Expanding Beyond Aortic Valve Boundaries
Global procedure volumes are surging, creating a robust environment for sustained expansion in the Cardiovascular devices market. Total surgical procedure volumes reached 18.9 million in 2024, with the United States and China accounting for 3.6 million and 3.5 million interventions, respectively. High procedure counts are now spilling over into the tricuspid sector, which was previously an underserved clinical niche. Abbott’s TriClip surpassed 10,000 implants globally by April 2024, signaling that the market is successfully moving beyond aortic valve replacements into complex mitral and tricuspid therapies. Manufacturers are unlocking entirely new revenue streams by addressing these untreated structural heart populations.
Lifecycle management of heart valves is emerging as a critical secondary growth driver in the cardiovascular devices market. Reintervention technologies are gaining traction, evidenced by 1,036 Valve-in-Valve (ViV) TAVR procedures performed in the US in 2024. Medicare data analyzed in 2024 identified a cumulative total of 2,374 redo TAVR procedures, with adoption accelerating. Germany remains a lead adopter, recording 23,752 TAVR procedures in the 2023-2024 period. Stakeholders are witnessing the formation of a “lifetime management” market where repeat interventions provide recurring revenue opportunities long after the initial implant.
Workforce Shortages Catalyzing Need for Automated Lab Efficiency
Severe labor constraints are forcing healthcare providers to prioritize automation, reshaping procurement criteria in the Cardiovascular devices market. A 2024 study highlighted that 46% of US counties operate without a single practicing cardiologist, while 86% of rural counties lack access entirely. Such shortages have created critical bottlenecks, with the UK cardiac waiting list swelling to 421,433 patients by May 2024 and Canadian wait times reaching 194 days. Consequently, hospitals are favoring devices that reduce procedure times and require fewer personnel. Efficiency is no longer a convenience; it is a prerequisite for reducing backlogs.
Infrastructure investments are rising to accommodate these efficiency demands. The US supported 4,300 operational cardiac catheterization labs in 2024, while the number of hybrid cath labs globally reached 2,400. Capacity expansion is particularly aggressive in smaller facilities, where cath lab capacity increased by 81% between 2020 and 2024. Healthcare networks are investing heavily to equip these new spaces with advanced tools that maximize limited staff bandwidth. The market is shifting toward integrated solutions that allow fewer specialists to treat a higher volume of patients.
Regulatory Speed and AI Integration Accelerating Commercial Pipelines
Regulatory bodies are actively removing barriers to entry, accelerating the time-to-revenue for novel technologies in the Cardiovascular devices market. The FDA listed 1,176 devices in its Breakthrough Device Program as of August 2024, with 243 designations specifically allocated to cardiovascular innovations—the highest of any clinical category. In the 12 months leading up to August 2024, the FDA cleared 16 distinct breakthrough cardiovascular devices. Regulators are clearly aligning with industry efforts to fast-track life-saving tools, reducing the financial risk associated with long approval timelines.
Artificial Intelligence is further streamlining the pathway from development to commercialization in the cardiovascular devices market. By May 2025, the FDA cleared a record 39 new clinical AI algorithms in a single month, bringing the total to 116 cleared cardiology algorithms. Major hardware approvals are benefitting from this favorable climate. Edwards Lifesciences received approval for the Evoque system in February 2024, followed by Abbott’s TriClip G4 in April 2024. These milestones signal a regulatory environment that encourages high-tech cardiac solutions, fostering a cycle of rapid innovation and market introduction.
Economic Pressures Reshaping Reimbursement and Procedure Cost Structures
Financial dynamics are driving a structural shift toward outpatient care settings within the Cardiovascular devices market. While the 2025 Medicare Physician Fee Schedule reduced the conversion factor by 2.83% to USD 32.35, payments to Ambulatory Surgical Centers (ASCs) were finalized to increase by 2.6%. Such divergent reimbursement policies incentivize the migration of routine procedures like stents and pacemakers to lower-cost ASC environments. Cost disparities further influence market behavior, as the average cost for a TAVR procedure in 2024 ranged from USD 52,176 in Chicago to USD 81,889 in California.
Global pricing variances are creating new patient mobility patterns affecting device consumption. Medical tourism options for TAVR in India were priced at approximately USD 14,500 in 2024, attracting self-pay patients from high-cost regions. Concurrently, payers are focusing on value-based care. CMS introduced new G-codes for 2025 to reimburse Atherosclerotic Cardiovascular Disease risk assessment specifically. Broader economic impacts are substantial, with projected global healthcare cost savings from wearable adoption estimated at USD 200 billion over the next 25 years. Efficient resource allocation is rapidly defining market winners and losers.
Consumer Wearables Merging with Clinical Diagnostic Workflows
Patient-generated data is evolving from a novelty into a critical input for the Cardiovascular devices market. Global shipments of wearable health devices reached 440 million units in 2024, with 160 million units specifically designated as medical sensors for clinical monitoring. The US market for smart wearable ECG monitors was valued at USD 586.91 million in 2024. These devices are effectively expanding the diagnostic funnel, identifying at-risk patients earlier and driving them into the professional care ecosystem. Approximately 225 million people globally utilized smartwatches with health-tracking features in 2024, creating a massive base for remote patient monitoring.
Usage patterns confirm that consumers are actively engaging with their cardiac health. A 2024 survey indicated that 92% of US smartwatch users utilize their device primarily for health tracking. Moreover, 37% of users specifically monitor heart metrics like heart rate, while 59% track steps for cardiovascular fitness. Clinicians are leveraging this trend to monitor patients remotely, reducing hospital readmissions. The market is successfully integrating consumer technologies into professional pathways, blurring the lines between lifestyle gadgets and medical-grade diagnostics.
Clinical Trial Ecosystem Powering Future Therapeutic Breakthroughs
Research activity is intensifying, acting as a leading indicator for future growth in the Cardiovascular devices market. A 2025 analysis identified 51 major cardiovascular clinical trials published between 2019 and 2024, enrolling a total of 292,985 patients. The immense scale of these studies creates a high barrier to entry for smaller competitors. The median number of sites for major trials was 320, with nearly one-third utilizing more than 500 distinct clinical sites in 2024. Only well-capitalized market leaders can sustain such extensive data generation efforts.
The innovation pipeline remains robust, promising a steady flow of new products. As of December 2025, over 2,000 cardiovascular drugs and device-delivery therapies were in active development. New clinical trial initiations surged by 68% in the decade ending 2024, reflecting strong R&D investment. Geographic diversity is also shifting strategies, as US sites enrolled only 19.9% of global participants in major cardiology studies analyzed in 2025. Companies are increasingly relying on global data to validate therapies, ensuring that new devices are approved for international markets simultaneously.
Manufacturing Investments Securing Supply Chain Against Global Demand
Strategic capital allocation is fortifying the supply chain, ensuring the Cardiovascular devices market can meet rising global demand. Abbott announced the construction of a new manufacturing facility in Georgia in 2025 to boost domestic output. Mergers and acquisitions are also focusing on securing proprietary technologies. Boston Scientific acquired the Silk Road Medical TCAR portfolio for USD 1.16 billion in 2024 to expand its vascular footprint. Domestic production capabilities remain vital, with 45% of global cath lab equipment exports originating from US manufacturers in 2024.
Investments are simultaneously flowing into digital infrastructure to modernize care delivery. Funding for AI-integrated cath lab solutions rose by 20% in 2024, aiming to improve procedural precision. Operational readiness is improving alongside these investments, with 29% more hybrid cath labs operational in 2024 compared to 2022. Financial backing remains steady in the cardiovascular devices market, with vascular device investment reaching USD 759 million and cardiovascular venture investment stabilizing at USD 542 million in 2024. Strong investor confidence confirms the long-term viability and profitability of the sector.
Tailor This Report to Your Specific Business Needs: https://www.astuteanalytica.com/ask-for-customization/cardiovascular-devices-market
Cardiovascular Devices Market Major Players:
- Abbott
- BIOTRONIK SE & Co. KG
- Boston Scientific Corporation
- Canon Medical Systems
- B. Braun SE
- Cardinal Health
- Medtronic
- LivaNova PLC
- Siemens Healthcare GmbH
- Edwards Lifesciences Corporation
- GE Healthcare
- W. L. Gore & Associates, Inc.
- Other Prominent Players
Key Market Segmentation:
By Device Type
- Diagnostic & Monitoring
- ECG
- Holter Monitors
- Event Monitors
- Implantable Loop Recorders
- Echocardiogram
- Pet Scan
- MRI
- Cardiac CT
- Doppler Fetal Monitors
- Therapeutic & Surgical Devices
- Pacemakers
- Stents
- Catheters and accessories
- Guidewires
- Cannulae
- Electrosurgical Procedures
- Valves
- Occlusion Devices
- Others
By Application
- Cardiac Arrhythmia
- Coronary Artery Disease
- Heart Failure
- Others
By End User
- Hospitals
- Specialty Clinics
- Others
By Region
- North America
- Europe
- Asia Pacific
- Middle East and Africa
- South America
Want Clarity on Report Coverage? Schedule a Quick Demo Call: https://www.astuteanalytica.com/report-walkthrough/cardiovascular-devices-market
About Astute Analytica
Astute Analytica is a global market research and advisory firm providing data-driven insights across industries such as technology, healthcare, chemicals, semiconductors, FMCG, and more. We publish multiple reports daily, equipping businesses with the intelligence they need to navigate market trends, emerging opportunities, competitive landscapes, and technological advancements.
With a team of experienced business analysts, economists, and industry experts, we deliver accurate, in-depth, and actionable research tailored to meet the strategic needs of our clients. At Astute Analytica, our clients come first, and we are committed to delivering cost-effective, high-value research solutions that drive success in an evolving marketplace.
Contact Us:
Astute Analytica
Phone: +1-888 429 6757 (US Toll Free); +91-0120- 4483891 (Rest of the World)
For Sales Enquiries: sales@astuteanalytica.com
Website: https://www.astuteanalytica.com/
Follow us on: LinkedIn | Twitter | YouTube
CONTACT: Contact Us: Astute Analytica Phone: +1-888 429 6757 (US Toll Free); +91-0120- 4483891 (Rest of the World) For Sales Enquiries: sales@astuteanalytica.com Website: https://www.astuteanalytica.com/

Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaShorts takes no editorial responsibility for the same.