Choroidal Neovascularization Market to Witness Accelerated Growth Throughout the Forecast Period (2025–2034) Due to Advancement in Anti-VEGF Therapies | DelveInsight
The choroidal neovascularization therapeutics market is expected to further increase due to major drivers, including the rising prevalence of the population, technological advancements, and upcoming therapies such as AbbVie and REGENXBIO’s ABBV-RGX-314, Adverum Biotechnologies’ Ixoberogene soroparvovec, Neuracle Genetics’ NG101, and others, during the forecast period (2025–2034).
New York, USA, Nov. 05, 2025 (GLOBE NEWSWIRE) — Choroidal Neovascularization Market to Witness Accelerated Growth Throughout the Forecast Period (2025–2034) Due to Advancement in Anti-VEGF Therapies | DelveInsight
The choroidal neovascularization therapeutics market is expected to further increase due to major drivers, including the rising prevalence of the population, technological advancements, and upcoming therapies such as AbbVie and REGENXBIO’s ABBV-RGX-314, Adverum Biotechnologies’ Ixoberogene soroparvovec, Neuracle Genetics’ NG101, and others, during the forecast period (2025–2034).
DelveInsight’s Choroidal Neovascularization Market Insights report includes a comprehensive understanding of current treatment practices, emerging choroidal neovascularization drugs, market share of individual therapies, and current and forecasted choroidal neovascularization market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
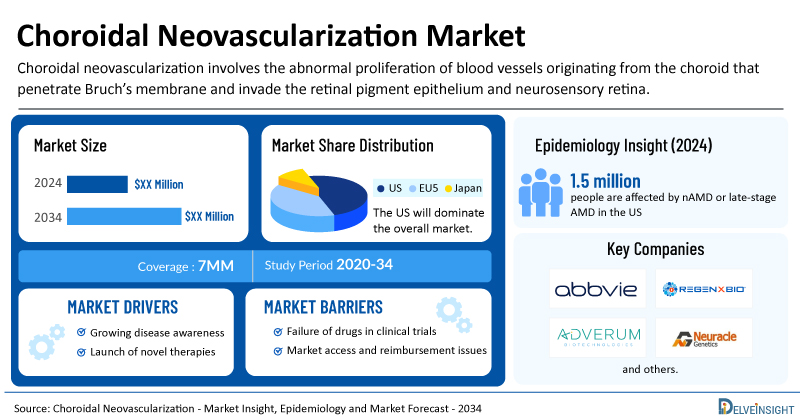
Choroidal Neovascularization Market Summary
- The total choroidal neovascularization treatment market size is expected to grow positively by 2034 in the leading markets.
- The United States accounts for the largest market size of choroidal neovascularization, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- In the US, around 1.5 million people are affected by nAMD or late-stage AMD, underscoring the major visual and functional burden of choroidal neovascularization.
- Key choroidal neovascularization companies, including AbbVie, REGENXBIO, Neuracle Genetics, Adverum Biotechnologies, and others, are actively working on innovative choroidal neovascularization drugs.
- Some of the key choroidal neovascularization therapies in clinical trials include Surabgene lomparvovec (ABBV-RGX-314), NG101, Ixoberogene soroparvovec, and others. These novel choroidal neovascularization therapies are anticipated to enter the choroidal neovascularization market in the forecast period and are expected to change the market.
Discover which choroidal neovascularization medications are expected to grab the market share @ Choroidal Neovascularization Market Report
Key Factors Driving the Growth of the Choroidal Neovascularization Market
Rising prevalence of AMD and aging populations
The global increase in age-related macular degeneration (the leading cause of CNV) and the expanding geriatric population are the primary demand drivers, with more patients, more diagnoses, and long-term treatment needs.
Improved diagnostics and screening
The wider adoption of high-resolution retinal imaging (OCT, OCT angiography) and remote screening programs increases early detection rates and referrals to treatment, thereby enlarging the treated population. This also enables treat-and-extend protocols that improve clinic throughput and adherence.
Launch of emerging choroidal neovascularization gene therapies
The anticipated launch of emerging choroidal neovascularization gene therapies, such as surabgene lomparvovec (ABBV-RGX-314) by AbbVie/REGENXBIO, NG101 by Neuracle Genetics, and Ixoberogene soroparvovec by Adverum Biotechnologies, among others, is expected to change the market dynamics.
Choroidal Neovascularization Market Analysis
Choroidal neovascularization is primarily treated using intravitreal anti-VEGF therapy, which inhibits abnormal blood vessel formation and leakage beneath the retina, key causes of vision impairment. Leading treatments include VABYSMO (faricimab-svoa) by Genentech, the first bispecific antibody targeting both VEGF-A and Ang-2, and BEOVU (brolucizumab-dbll) by Novartis, developed for prolonged efficacy with extended dosing intervals. These therapies have demonstrated notable improvements in visual acuity and a reduction in retinal fluid.
Next-generation candidates such as surabgene lomparvovec (ABBV-RGX-314) by AbbVie/REGENXBIO, Ixoberogene soroparvovec by Adverum Biotechnologies, and NG101 by Neuracle Genetics are designed to deliver long-term VEGF inhibition through single-dose administration, potentially easing the treatment burden. Complementary options, including laser photocoagulation and photodynamic therapy, remain applicable in selected cases.
Together, these innovations are transforming disease management, shifting from frequent injections to durable, mechanism-based therapies that sustain vision over time. With ongoing research and innovation, the outlook for choroidal neovascularization is increasingly optimistic, with the 7MM choroidal neovascularization market expected to undergo substantial change between 2025 and 2034.
Learn more about the choroidal neovascularization treatment options @ Choroidal Neovascularization Treatment Market
Choroidal Neovascularization Competitive Landscape
Emerging choroidal neovascularization gene therapies, such as surabgene lomparvovec (ABBV-RGX-314) by AbbVie/REGENXBIO, NG101 by Neuracle Genetics, and Ixoberogene soroparvovec by Adverum Biotechnologies, are driving a shift toward durable, one-time treatments for choroidal neovascularization, aiming to address the root causes, reduce treatment burden, and enable lasting vision preservation.
AbbVie and REGENXBIO’s Surabgene lomparvovec (ABBV-RGX-314) is an experimental gene therapy targeting choroidal neovascularization in neovascular age-related macular degeneration (nAMD). It employs an adeno-associated virus (AAV8) vector to deliver a gene encoding an antibody fragment that inhibits VEGF production within retinal cells. Designed as a one-time treatment, it enables continuous intraocular anti-VEGF expression, potentially reducing the frequency of injections and offering durable disease control and vision preservation.
Neuracle Genetics’ NG101 is another investigational gene therapy in development for choroidal neovascularization associated with nAMD. The therapy delivers a therapeutic gene to retinal cells, blocking abnormal blood vessel growth and maintaining retinal integrity. By addressing the molecular mechanisms driving disease progression, NG101 aims to provide a long-term, single-administration solution that sustains vision and minimizes the need for repeated intravitreal injections.
Adverum Biotechnologies’ Ixoberogene soroparvovec is a one-time gene therapy candidate for choroidal neovascularization due to nAMD. It utilizes a proprietary AAV.7m8 vector to introduce a gene encoding an anti-VEGF protein directly into retinal cells, thereby promoting sustained intraocular expression and inhibiting pathological angiogenesis and vascular leakage. This approach aims to achieve long-term disease suppression, reduce or eliminate the need for frequent injections, and preserve visual function over time.
The anticipated launch of these emerging choroidal neovascularization therapies are poised to transform the choroidal neovascularization market landscape in the coming years. As these cutting-edge choroidal neovascularization therapies continue to mature and gain regulatory approval, they are expected to reshape the choroidal neovascularization market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for choroidal neovascularization, visit @ Choroidal Neovascularization Medication
What is Choroidal Neovascularization?
Choroidal neovascularization involves the abnormal proliferation of blood vessels originating from the choroid that penetrate Bruch’s membrane and invade the retinal pigment epithelium and neurosensory retina. This process leads to fluid accumulation, hemorrhage, and fibrotic scarring, often causing permanent loss of central vision. CNV most frequently arises as a complication of neovascular age-related macular degeneration (nAMD) and pathological myopia, both of which are leading global causes of visual impairment. Affected individuals commonly report blurred or distorted central vision, metamorphopsia, and the presence of dark or empty areas in their sight. The pathogenesis is primarily driven by the upregulation of vascular endothelial growth factor (VEGF) expression, induced by hypoxia, inflammation, and oxidative stress, which collectively promote aberrant angiogenesis and increased vascular permeability.
Choroidal Neovascularization Epidemiology Segmentation
The choroidal neovascularization epidemiology section provides insights into the historical and current choroidal neovascularization patient pool and forecasted trends for the leading markets. In Japan, the prevalence of nAMD is estimated at 0.52%, while myopic choroidal neovascularization accounts for approximately 11.3% of total cases of high myopia. This distribution reflects the distinct regional pattern of choroidal neovascularization, where high myopia contributes significantly to disease burden, underscoring the importance of early identification and long-term monitoring in at-risk populations.
The choroidal neovascularization market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- Total Prevalent Cases of Pathological Myopia
- Total Prevalent Cases of AMD
- Total Diagnosed Prevalent Cases of Choroidal Neovascularization
- Gender-Specific Diagnosed Prevalent Cases of Choroidal Neovascularization
- Age-Specific Diagnosed Prevalent Cases of Choroidal Neovascularization
- Treated Cases of Choroidal Neovascularization
Download the report to understand choroidal neovascularization management @ Choroidal Neovascularization Treatment Options
| Choroidal Neovascularization Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Choroidal Neovascularization Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Choroidal Neovascularization Epidemiology Segmentation | Total Prevalent Cases of Pathological Myopia, Total Prevalent Cases of AMD, Total Diagnosed Prevalent Cases of Choroidal Neovascularization, Gender-Specific Diagnosed Prevalent Cases of Choroidal Neovascularization, Age-Specific Diagnosed Prevalent Cases of Choroidal Neovascularization, and Treated Cases of Choroidal Neovascularization |
| Key Choroidal Neovascularization Companies | AbbVie, REGENXBIO, Neuracle Genetics, Adverum Biotechnologies, Genentech, Chugai Pharmaceutical, Novartis, and others |
| Key Choroidal Neovascularization Therapies | Surabgene lomparvovec (ABBV-RGX-314), NG101, Ixoberogene soroparvovec, VABYSMO, BEOVU, and others |
Scope of the Choroidal Neovascularization Market Report
- Choroidal Neovascularization Therapeutic Assessment: Choroidal Neovascularization current marketed and emerging therapies
- Choroidal Neovascularization Market Dynamics: Conjoint Analysis of Emerging Choroidal Neovascularization Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Choroidal Neovascularization Market Unmet Needs, KOL’s views, Analyst’s views, Choroidal Neovascularization Market Access and Reimbursement
Discover more about choroidal neovascularization drugs in development @ Choroidal Neovascularization Clinical Trials
Table of Contents
| 1 | Choroidal Neovascularization Market Key Insights |
| 2 | Choroidal Neovascularization Market Report Introduction |
| 3 | Choroidal Neovascularization Market Overview at a Glance |
| 3.1 | Market Share (%) Distribution of Choroidal Neovascularization by Therapies in the 7MM in 2024 |
| 3.2 | Market Share (%) Distribution of Choroidal Neovascularization by Therapies in the 7MM in 2034 |
| 4 | Epidemiology and Market Methodology |
| 5 | Executive Summary |
| 6 | Key Events |
| 7 | Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | Choroidal Neovascularization Types |
| 7.3 | Choroidal Neovascularization Causes |
| 7.4 | Choroidal Neovascularization Pathophysiology |
| 7.5 | Chorodial Neovascularization Symptoms |
| 7.6 | Choroidal Neovascularization Risk Factor |
| 7.7 | Choroidal Neovascularization Diagnosis |
| 7.8 | Choroidal Neovascularization Treatment and Management |
| 8 | Epidemiology and Patient Population |
| 8.1 | Key Findings |
| 8.2 | Assumptions and Rationale: 7MM |
| 8.2.1 | Total Prevalent Cases of Pathological Myopia |
| 8.2.2 | Total Prevalent Cases of AMD |
| 8.2.3 | Total Diagnosed Prevalent Cases of Choroidal Neovascularization |
| 8.2.4 | Gender-Specific Diagnosed Prevalent Cases of Choroidal Neovascularization |
| 8.2.5 | Age-Specific Diagnosed Prevalent Cases of Choroidal Neovascularization |
| 8.3 | Total Diagnosed Prevalent Cases of Choroidal Neovascularization in the 7MM |
| 8.4 | The United States |
| 8.5 | EU4 and the UK |
| 8.6 | Japan |
| 9 | Choroidal Neovascularization Patient Journey |
| 10 | Marketed Choroidal Neovascularization Therapies |
| 10.1 | Key Cross Competition |
| 10.2 | VABYSMO (faricimab-svoa): Genentech/ Chugai Pharmaceutical |
| 10.2.1 | Product Description |
| 10.2.2 | Regulatory Milestones |
| 10.2.3 | Other Development Activities |
| 10.2.4 | Clinical Trials Information |
| 10.2.5 | Safety and Efficacy |
| 10.3 | BEOVU (brolucizumab-dbll): Novartis |
| The list will be continued in the report | |
| 11 | Emerging Choroidal Neovascularization Therapies |
| 11.1 | Key Cross Competition |
| 11.2 | Surabgene lomparvovec (ABBV-RGX-314): AbbVie/ REGENXBIO |
| 11.2.1 | Drug Description |
| 11.2.2 | Other Development Activities |
| 11.2.3 | Clinical Trials Information |
| 11.2.4 | Safety and Efficacy |
| 11.2.5 | Analyst’s View |
| 11.3 | NG101: Neuracle Genetics |
| 11.4 | Ixoberogene soroparvovec: Adverum Biotechnologies |
| The list will be continued in the report | |
| 12 | Choroidal Neovascularization Market: Seven Major Market Analysis |
| 12.1 | Key Findings |
| 12.2 | Choroidal Neovascularization Market Outlook |
| 12.3 | Attribute Analysis |
| 12.4 | Key Choroidal Neovascularization Market Forecast Assumptions |
| 12.5 | Total Market Size of Choroidal Neovascularization in the 7MM |
| 12.6 | Market Size of Choroidal Neovascularization by Therapies in the 7MM |
| 12.7 | The United States Choroidal Neovascularization Market Size |
| 12.7.1 | Total Market Size of Choroidal Neovascularization |
| 12.7.2 | Market Size of Choroidal Neovascularization by Therapies in the US |
| 12.8 | EU4 and the UK Choroidal Neovascularization Market Size |
| 12.9 | Japan Choroidal Neovascularization Market Size |
| 13 | Key Opinion Leaders’ Views |
| 14 | Choroidal Neovascularization Market Unmet Needs |
| 15 | Choroidal Neovascularization Market SWOT Analysis |
| 16 | Choroidal Neovascularization Market Access and Reimbursement |
| 17 | Bibliography |
| 18 | Abbreviations and Acronyms |
| 18 | Choroidal Neovascularization Market Report Methodology |
Related Reports
Choroidal Neovascularization Clinical Trial Analysis
Choroidal Neovascularization Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key choroidal neovascularization companies, including AbbVie, REGENXBIO, Neuracle Genetics, Adverum Biotechnologies, Genentech, Chugai Pharmaceutical, Novartis, among others.
Age-related Macular Degeneration Market
Age-related Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key AMD companies, including Opthea Limited, Kodiak Sciences Inc., REGENXBIO, Alkahest Inc, Evergreen Therapeutics, Alkeus Pharmaceuticals, Ribomic USA Inc, Outlook Therapeutics, Inc., Unity Biotechnology, Inc, PanOptica, Inc., Clearside Biomedical, Alexion Pharmaceuticals, AstraZeneca, Alkeus Pharmaceuticals, Stealth BioTherapeutics, CellCure Neurosciences, Regenerative Patch Technologies, Allegro Ophthalmics, Annexon Biosciences, NGM Biopharmaceuticals, Ionis Pharmaceuticals, Apellis Pharmaceuticals, Iveric Bio, Gyroscope Therapeutics, Novartis, Luxa Biotechnology, Gemini Therapeutics, among others.
Age-related Macular Degeneration Clinical Trial Analysis
Age-related Macular Degeneration Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key age-related macular degeneration companies including Regeneron Pharmaceuticals, Novartis, Roche, Opthea Limited, Kodiak Sciences Inc., REGENXBIO, Alkahest Inc, Graybug Vision, Ribomic USA Inc, Outlook Therapeutics, Inc., Unity Biotechnology, Inc, PanOptica, Inc., Clearside Biomedical, Alexion Pharmaceuticals, AstraZeneca, Evergreen Therapeutics, Alkeus Pharmaceuticals, Stealth BioTherapeutics, CellCure Neurosciences, Regenerative Patch Technologies, Allegro Ophthalmics, Annexon Biosciences, NGM Biopharmaceuticals, Ionis Pharmaceuticals, Apellis Pharmaceuticals, Iveric Bio, Gyroscope Therapeutics, Luxa Biotechnology, Gemini Therapeutics, among others.
Dry Age-related Macular Degeneration Market
Dry Age-related Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Dry AMD companies including Molecular Partners, Stealth BioTherapeutics, Regenerative Patch Technologies, Aevitas Therapeutics, NGM Biopharmaceuticals, InflammX Therapeutics, Lineage Cell Therapeutics, Alexion AstraZeneca Rare Disease, Belite Bio, Katairo, Cognition Therapeutics, Apellis Pharmaceuticals, Galimedix Therapeutics, Amarna Therapeutics, 4D Molecular Therapeutics, Aviceda Therapeutics, Isarna Therapeutics, among others.
Wet Age-related Macular Degeneration Market
Wet Age-related Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key wet AMD companies including EyePoint Pharmaceuticals, Inc., AbbVie, Caregen Co. Ltd., Exegenesis Bio, Shanghai Henlius Biotech, Skyline Therapeutics, 4D Molecular Therapeutics, Ocugenix Corporation, Adverum Biotechnologies, Inc., Ashvattha Therapeutics, Inc., AiViva BioPharma, Inc., Ocular Therapeutix, Inc., Clearside Biomedical, Inc., Hoffmann-La Roche, Kyowa Kirin, Inc., Opthea Limited, AffaMed Therapeutics Limited, EyeBiotech Ltd., Novartis, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com

Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaShorts takes no editorial responsibility for the same.
