
The gene therapy market for ocular rare diseases is expanding rapidly as breakthroughs in viral vectors (like AAV) and gene-editing tools enable one-time, disease-modifying treatments for previously untreatable inherited retinal disorders. Additionally, the launch of emerging therapies such as MCO-010 (Nanoscope/Verana Health), SPVN06 (SparingVision), OCU400 (Ocugen), laru-zova (Beacon Therapeutics), botaretigene sparoparvovec (Johnson & Johnson/MeiraGTx), HORA-PDE6B (eyeDNA Therapeutics/Coave Therapeutics), and others will further fuel the market growth.
New York, USA, Nov. 19, 2025 (GLOBE NEWSWIRE) — Gene Therapy for Ocular Rare Disease Market to Exhibit Phenomenal Growth During the Forecast Period (2025–2034) Due to Expanding Pipeline and Clinical Trial Activities | DelveInsight
The gene therapy market for ocular rare diseases is expanding rapidly as breakthroughs in viral vectors (like AAV) and gene-editing tools enable one-time, disease-modifying treatments for previously untreatable inherited retinal disorders. Additionally, the launch of emerging therapies such as MCO-010 (Nanoscope/Verana Health), SPVN06 (SparingVision), OCU400 (Ocugen), laru-zova (Beacon Therapeutics), botaretigene sparoparvovec (Johnson & Johnson/MeiraGTx), HORA-PDE6B (eyeDNA Therapeutics/Coave Therapeutics), and others will further fuel the market growth.
DelveInsight’s Gene Therapy for Ocular Rare Disease Market Insights report includes a comprehensive understanding of current treatment practices, emerging gene therapy for ocular rare disease, market share of individual therapies, and current and forecasted emerging gene therapy for ocular rare disease market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
Gene Therapy for Ocular Rare Disease Market Summary
- The total gene therapy for ocular rare disease treatment market size is expected to grow positively by 2034 in the leading markets.
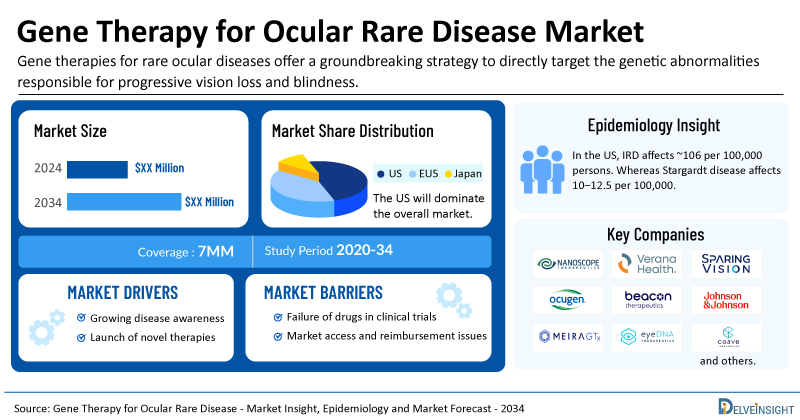
- The United States accounts for the largest market size of gene therapy for ocular rare disease, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- Inherited retinal diseases (IRD) represent a group of rare disorders that include conditions such as achromatopsia, choroideremia, congenital night blindness, and retinitis pigmentosa (pigmentary retinal dystrophy).
- Key gene therapy for ocular rare disease companies, including Nanoscope, Verana Health, SparingVision, Ocugen, Beacon Therapeutics, Johnson & Johnson, MeiraGTx, eyeDNA Therapeutics, Coave Therapeutics, Atsena Therapeutics, Opus Genetics, Sepul Bio, Théa, Editas Medicine, SpliceBio, VeonGen, Ascidian Therapeutics, GenSight Biologics, and others, are actively working on innovative gene therapy for ocular rare disease drugs.
- Some of the key gene therapies for ocular rare disease in clinical trials include MCO-010, SPVN06, OCU400, laru-zova, botaretigene sparoparvovec, HORA-PDE6B, ATSN-101, AAV-AIPL1, AAV-AIPL1, OPGx-LCA5, Sepofarsen, EDIT-101, SB-007, VG801, ACDN-01, GS030, and others. These novel gene therapy for ocular rare disease are anticipated to enter the gene therapy for ocular rare disease market in the forecast period and are expected to change the market.
Discover which gene therapy for ocular rare disease medications are expected to grab the market share @ Gene Therapy for Ocular Rare Disease Market Report
Key Factors Driving the Growth of the Gene Therapy for Ocular Rare Disease Market
Rapid advances in vector and gene-editing technologies
Continuous innovation in adeno-associated virus (AAV) vector design, including next-generation capsids with enhanced tropism for retinal cells, has significantly improved transduction efficiency and safety profiles. Non-viral platforms such as lipid nanoparticles and exosome-based delivery are emerging as promising alternatives to overcome immune and packaging limitations. Moreover, precision-editing tools like CRISPR/Cas9, base editors, and prime editors are expanding the scope of treatable mutations and enabling permanent correction at the genomic level.
Better genetic testing and diagnosis
The widespread adoption of next-generation sequencing (NGS), whole-exome, and whole-genome sequencing has significantly enhanced the ability to identify causative mutations in rare ocular diseases. Early and precise molecular diagnosis enables clinicians to match patients with the most appropriate gene therapy candidates and facilitates stratified clinical trial recruitment.
Rising gene therapy for ocular rare disease clinical trial activities
Several gene therapies targeting rare ocular diseases are currently in clinical trials, including MCO-010 (Nanoscope/Verana Health), SPVN06 (SparingVision), OCU400 (Ocugen), laru-zova (Beacon Therapeutics), botaretigene sparoparvovec (Johnson & Johnson/MeiraGTx), HORA-PDE6B (eyeDNA Therapeutics/Coave Therapeutics), ATSN-101 (Atsena Therapeutics), AAV-AIPL1 (MeiraGTx), OPGx-LCA5 (Opus Genetics), Sepofarsen (Sepul Bio/Théa), EDIT-101 (Editas Medicine), SB-007 (SpliceBio), VG801 (VeonGen), ACDN-01 (Ascidian Therapeutics), GS030 (GenSight Biologics), and others.
Gene Therapy for Ocular Rare Disease Market Analysis
Gene therapies for rare ocular diseases aim to correct defective genes in the eye, thereby preserving or restoring vision. Their goal is to slow or stop disease progression, enhance visual function, and provide durable benefits that go beyond conventional treatments.
The treatment landscape for rare eye disorders is rapidly evolving, with approved therapies such as LUXTURNA (voretigene neparvovec-rzyl) from Spark Therapeutics/Novartis and ENCELTO (revakinagene taroretcel-lwey) from Neurotech Pharmaceuticals, both of which address the genetic root causes of vision loss and offer lasting, disease-modifying outcomes.
The development pipeline is also expanding with next-generation candidates, including MCO-010 (Nanoscope/Verana Health), SPVN06 (SparingVision), and OCU400 (Ocugen). These investigational therapies target key genetic mechanisms driving retinal degeneration and aim to deliver sustained therapeutic effects beyond existing modalities.
However, significant challenges remain, including limited mutation coverage, uncertain long-term durability, complex delivery to retinal tissue, high treatment costs, and restricted access, leaving many patients still without effective gene-based solutions.
Learn more about the gene therapy for ocular rare disease treatment options @ Gene Therapy for Ocular Rare Disease Treatment Market
Gene Therapy for Ocular Rare Disease Competitive Landscape
Some of the gene therapies for ocular rare disease in clinical trials include MCO-010 (Nanoscope/Verana Health), SPVN06 (SparingVision), OCU400 (Ocugen), laru-zova (Beacon Therapeutics), botaretigene sparoparvovec (Johnson & Johnson/MeiraGTx), HORA-PDE6B (eyeDNA Therapeutics/Coave Therapeutics), ATSN-101 (Atsena Therapeutics), AAV-AIPL1 (MeiraGTx), OPGx-LCA5 (Opus Genetics), Sepofarsen (Sepul Bio/Théa), EDIT-101 (Editas Medicine), SB-007 (SpliceBio), VG801 (VeonGen), ACDN-01 (Ascidian Therapeutics), GS030 (GenSight Biologics), and others.
MCO-010, developed by Nanoscope Therapeutics in collaboration with Verana Health, is an experimental gene therapy targeting inherited retinal diseases (IRDs), including retinitis pigmentosa and Stargardt disease. The therapy employs an optogenetic adeno-associated virus (AAV) vector to introduce a gene that encodes a light-sensitive protein into retinal cells. This approach aims to re-activate dormant photoreceptors, restore visual function, and deliver long-lasting, disease-modifying benefits that go beyond traditional treatment methods.
SparingVision’s SPVN06 is another investigational AAV-based gene therapy under development for IRDs driven by photoreceptor degeneration. It is designed to preserve cone function and safeguard the remaining retinal cells, directly addressing the root cause of vision loss rather than merely alleviating symptoms. By targeting disease mechanisms, SPVN06 has the potential to slow or prevent disease progression, sustain visual capability, and improve long-term outcomes—marking a transition toward more durable, disease-modifying therapies for rare ocular disorders.
Ocugen’s OCU400 is an investigational gene therapy aimed at treating inherited retinal diseases through delivery of a functional NR2E3 gene using an AAV vector. This approach seeks to regulate multiple retinal pathways and restore balance within degenerating photoreceptors. By addressing the underlying molecular dysfunction, OCU400 aspires to provide sustained, disease-modifying effects and serve as a transformative alternative to conventional symptom-focused treatments.
The anticipated launch of these emerging gene therapies for ocular rare disease are poised to transform the gene therapy for ocular rare disease market landscape in the coming years. As these cutting-edge gene therapies for ocular rare diseases continue to mature and gain regulatory approval, they are expected to reshape the gene therapy for ocular rare disease market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new gene therapy for ocular rare disease, visit @ Gene Therapy for Ocular Rare Disease Medication
Recent Developments in the Gene Therapy for Ocular Rare Disease Market
- In September 2025, Nanoscope Therapeutics reported that it had strengthened global regulatory pathways for its investigational gene therapy MCO-010 by securing an FDA RMAT designation and five EMA Orphan Drug Designation (ODD), underscoring its potential in treating IRD.
- In August 2025, a US patient received the world’s first administration of ENCELTO (revakinagene taroretcel-lwey) gene therapy for MacTel, marking a historic milestone in ocular rare disease treatment.
- In August 2025, Nanoscope Therapeutics reported groundbreaking three-year data from its REMAIN trial for patients with retinitis pigmentosa, presented at the 2025 Euretina Congress and the Retina Society Annual Scientific Meeting, highlighting the long-term benefits of its investigational gene therapy MCO-010.
- In May 2025, SparingVision reported a favorable safety update from its PRODYGY trial of SPVN06 at the ARVO 2025 meeting, supporting the continued clinical development of its gene therapy for IRD.
- In March 2025, Neurotech’s ENCELTO (revakinagene taroretcel-lwey) was approved by the US FDA for the treatment of macular telangiectasia type 2 (MacTel), making it the first FDA-approved therapy for this condition.
What is Gene Therapy for Ocular Rare Disease?
Gene therapies for rare ocular diseases offer a groundbreaking strategy to directly target the genetic abnormalities responsible for progressive vision loss and blindness. These treatments focus on inherited retinal disorders such as retinitis pigmentosa, Leber congenital amaurosis, and Stargardt disease, aiming to repair or replace defective genes within the eye. Clinical presentations can range from initial symptoms, such as night blindness, sensitivity to light, or peripheral vision loss, to more severe stages involving significant visual decline or total blindness. Although each condition stems from unique genetic origins, shared pathological mechanisms, such as photoreceptor degeneration, disrupted visual cycle processes, and retinal cell death, contribute to disease progression. By acting at the molecular level, gene therapies hold the promise of long-lasting restoration or preservation of vision. Early genetic screening and prompt therapeutic intervention are vital, as untreated progression can lead to irreversible blindness and a major reduction in quality of life.
Gene Therapy for Ocular Rare Disease Epidemiology Segmentation
The gene therapy for ocular rare disease epidemiology section provides insights into the historical and current gene therapy for ocular rare disease patient pool and forecasted trends for the leading markets. In the US, IRD affects approximately 106 per 100,000 persons. Whereas Stargardt disease affects 10–12.5 per 100,000. This distribution underscores the heterogeneity of ocular rare diseases and highlights the disproportionate burden of IRD within the overall landscape.
The gene therapy for ocular rare disease market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- Type- specific Prevalent Cases of Ocular Rare Disease
- Total Diagnosed Prevalent Cases of Ocular Rare Disease
- Total Treated Cases of Ocular Rare Disease
Download the report to understand gene therapy for ocular rare disease management @ Gene Therapy for Ocular Rare Disease Treatment Options
| Gene Therapy for Ocular Rare Disease Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Gene Therapy for Ocular Rare Disease Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Gene Therapy for Ocular Rare Disease Epidemiology Segmentation | Type- specific Prevalent Cases of Ocular Rare Disease, Total Diagnosed Prevalent Cases of Ocular Rare Disease, Total Treated Cases of Ocular Rare Disease |
| Key Gene Therapy for Ocular Rare Disease Companies | Nanoscope, Verana Health, SparingVision, Ocugen, Beacon Therapeutics, Johnson & Johnson, MeiraGTx, eyeDNA Therapeutics, Coave Therapeutics, Atsena Therapeutics, Opus Genetics, Sepul Bio, Théa, Editas Medicine, SpliceBio, VeonGen, Ascidian Therapeutics, GenSight Biologics, Spark Therapeutics, Novartis, Neurotech Pharmaceuticals, and others |
| Key Gene Therapy for Ocular Rare Disease Therapies | MCO-010, SPVN06, OCU400, laru-zova, botaretigene sparoparvovec, HORA-PDE6B, ATSN-101, AAV-AIPL1, AAV-AIPL1, OPGx-LCA5, Sepofarsen, EDIT-101, SB-007, VG801, ACDN-01, GS030, LUXTURNA, ENCELTO, and others |
Scope of the Gene Therapy for Ocular Rare Disease Market Report
- Gene Therapy for Ocular Rare Disease Therapeutic Assessment: Gene Therapy for Ocular Rare Disease current marketed and emerging therapies
- Gene Therapy for Ocular Rare Disease Market Dynamics: Conjoint Analysis of Emerging Gene Therapy for Ocular Rare Disease Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Gene Therapy for Ocular Rare Disease Market Unmet Needs, KOL’s views, Analyst’s views, Gene Therapy for Ocular Rare Disease Market Access and Reimbursement
Discover more about gene therapy for ocular rare disease in development @ Gene Therapy for Ocular Rare Disease Clinical Trials
Table of Contents
| 1 | Gene Therapy for Ocular Rare Disease Market Key Insights |
| 2 | Gene Therapy for Ocular Rare Disease Market Report Introduction |
| 3 | Gene Therapy for Ocular Rare Disease Market Overview at a Glance |
| 3.1 | Gene Therapy for Ocular Rare Disease Market Share (%) Distribution by Therapies in the 7MM in 2024 |
| 3.2 | Gene Therapy for Ocular Rare Disease Market Share (%) Distribution by Therapies in the 7MM in 2034 |
| 4 | Epidemiology and Market Methodology |
| 5 | Executive Summary |
| 6 | Key Events |
| 7 | Disease Background and Overview |
| 8 | Epidemiology and Patient Population |
| 8.1 | Key Findings |
| 8.2 | Assumptions and Rationale: 7MM |
| 8.2.1 | Type-specific Prevalent Cases of Ocular Rare Disease |
| 8.2.2 | Diagnosed Prevalent Cases of Ocular Rare Disease |
| 8.3 | Total Diagnosed Prevalent Cases of Ocular Rare Disease in the 7MM |
| 8.4 | The United States |
| 8.5 | EU4 and the UK |
| 8.6 | Japan |
| 9 | Gene Therapy for Ocular Rare Disease Patient Journey |
| 10 | Marketed Gene Therapy for Ocular Rare Disease |
| 10.1 | Key Cross Competition |
| 10.2 | LUXTURNA (voretigene neparvovec-rzyl): SPARK THERAPEUTICS/ Novartis |
| 10.2.1 | Product Description |
| 10.2.2 | Regulatory Milestones |
| 10.2.3 | Other Development Activities |
| 10.2.4 | Clinical Trials Information |
| 10.2.5 | Safety and Efficacy |
| 10.3 | ENCELTO (revakinagene taroretcel-lwey): Neurotech Pharmaceuticals |
| 11 | Emerging Gene Therapy for Ocular Rare Disease |
| 11.1 | Key Cross Competition |
| 11.2 | MCO-010: Nanoscope/Verana Health |
| 11.2.1 | Drug Description |
| 11.2.2 | Other Development Activities |
| 11.2.3 | Clinical Trials Information |
| 11.2.4 | Safety and Efficacy |
| 11.2.5 | Analyst’s View |
| 11.3 | SPVN06: SparingVision |
| 11.4 | OCU400: Ocugen |
| The list will be continued in the report | |
| 12 | Gene Therapy for Ocular Rare Disease Market: Seven Major Market Analysis |
| 12.1 | Key Findings |
| 12.2 | Gene Therapy for Ocular Rare Disease Market Outlook |
| 12.3 | Attribute Analysis |
| 12.4 | Key Gene Therapy for Ocular Rare Disease Market Forecast Assumptions |
| 12.5 | Total Market Size of Gene Therapy for Ocular Rare Diseases in the 7MM |
| 12.6 | Market Size of Gene Therapy for Ocular Rare Disease by Therapies in the 7MM |
| 12.7 | The United States Gene Therapy for Ocular Rare Disease Market Size |
| 12.8 | EU4 and the UK Gene Therapy for Ocular Rare Disease Market Size |
| 12.9 | Japan Gene Therapy for Ocular Rare Disease Market Size |
| 13 | Key Opinion Leaders’ Views on Gene Therapy for Ocular Rare Disease |
| 14 | Gene Therapy for Ocular Rare Disease Market Unmet Needs |
| 15 | Gene Therapy for Ocular Rare Disease Market SWOT Analysis |
| 16 | Gene Therapy for Ocular Rare Disease Market Access and Reimbursement |
| 17 | Bibliography |
| 18 | Abbreviations and Acronyms |
| 19 | Gene Therapy for Ocular Rare Disease Market Report Methodology |
Related Reports
Gene Therapies in Ophthalmology Market
Gene Therapies in Ophthalmology Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key gene therapies in ophthalmology companies, including BEACON THERAPEUTICS, NANOSCOPE THERAPEUTICS, COAVE THERAPEUTICS, BIONIC SIGHT, GENSIGHT BIOLOGICS, ADVERUM BIOTECHNOLOGIES, EYEVENSYS, EXEGENESIS BIO, MEIRAGTX, JOHNSON & JOHNSON INNOVATIVE MEDICINE, NEUROPHTH THERAPEUTICS, 4D MOLECULAR THERAPEUTICS, ATSENA THERAPEUTICS, OCUGEN, ABBVIE, REGENXBIO, SKYLINE THERAPEUTICS, HUIDAGENE THERAPEUTICS, OPUS GENETICS, among others.
Gene Therapies in Ophthalmology Competitive Landscape
Gene Therapies in Ophthalmology Competitive Landscape – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key gene therapies in ophthalmology companies, including Spark Therapeutics, Regenxbio, Beacon Therapeutics, Adverum Biotechnologies, Exegenesis Bio, Frontera Therapeutics, HuidaGene Therapeutics, Nanjing IASO Biotherapeutics, GenSight Biologics, Sylentis, Neurophth Therapeutics, Johnson & Johnson Innovative Medicine, Nanoscope Therapeutics, Eyevensys, Atsena Therapeutics Inc., Coave Therapeutics, OCUGEN, INC, Visgenx, Amarna Therapeutics, Ikarovec, Homology Medicines, Ray Therapeutics, Shanghai Refreshgene Technology Co., Ltd., Complement Therapeutics, Abeona Therapeutics, among others.
Gene Therapy Competitive Landscape
Gene Therapy Competitive Landscape – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key gene therapy companies, including Novartis, Johnson & Johnson, Fibrocell Technologies, Pfizer, HELIXMITH Co., Ltd., Sarepta Therapeutics, REGENXBIO, Solid Biosciences Inc., Lexeo Therapeutics, Spark Therapeutics, Xalud Therapeutics, uniQure, Ultragenyx Pharmaceutical, Nanoscope Therapeutics, among others.
Retinitis Pigmentosa Market
Retinitis Pigmentosa Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key gene therapy in retinitis pigmentosa companies, including Johnson & Johnson Innovative Medicine, MeiraGTx, Beacon Therapeutics, Nanoscope Therapeutics, Gensight Biologics, 4D Molecular Therapeutics, Coave Therapeutics, Ocugen, Bionic Sight, jCyte, Endogena Therapeutics, ProQR Therapeutics, Aldeyra Therapeutics, among others.
Stargardt Disease Market
Stargardt Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Stargardt disease companies, including Kubota Pharmaceuticals, Nanoscope Therapeutics, Alkeus Pharmaceuticals, Belite Bio, Astellas Pharma, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com

Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaShorts takes no editorial responsibility for the same.
