Global Healthcare Regulatory Affairs Outsourcing Market to Expand at 9.76% CAGR as Demand for Specialized Compliance, Faster Approvals, and Cost Optimization Accelerates.
Austin, Texas, Jan. 27, 2026 (GLOBE NEWSWIRE) — Healthcare Regulatory Affairs Outsourcing Market Size & Growth Analysis:
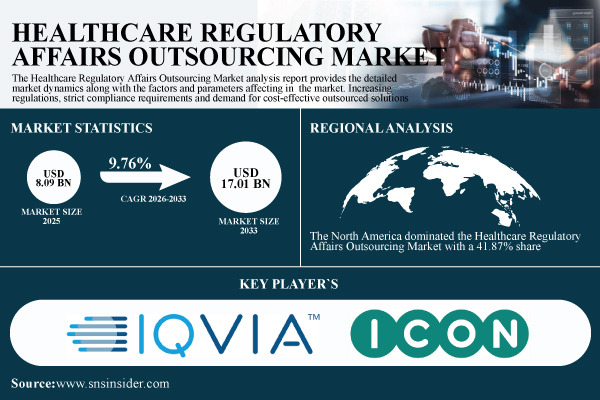
According to SNS Insider, the Healthcare Regulatory Affairs Outsourcing Market was valued at USD 8.09 billion in 2025 and is projected to reach USD 17.01 billion by 2033, expanding at a compound annual growth rate (CAGR) of 9.76% during the forecast period 2026–2033. The market is witnessing robust growth as pharmaceutical, biotechnology, and medical device companies increasingly rely on external regulatory expertise to navigate complex and evolving global compliance frameworks.
Market Size and Forecast:
- Market Size in 2025: USD 8.09 Billion
- Market Size by 2033: USD 17.01 Billion
- CAGR: 9.76% from 2026 to 2033
- Base Year: 2025
- Forecast Period: 2026–2033
- Historical Data: 2022–2024

Get a Sample Report of Healthcare Regulatory Affairs Outsourcing Market: https://www.snsinsider.com/sample-request/9049
The U.S. Healthcare Regulatory Affairs Outsourcing Market is projected to grow from USD 2.39 billion in 2025E to USD 4.37 billion by 2033, registering a CAGR of 7.86%. Growth is driven by rising regulatory scrutiny, increasing clinical trial activity, and the need for accelerated drug and device approvals. The presence of a strong pharmaceutical and biotechnology ecosystem, along with the rapid adoption of digital and AI-enabled regulatory solutions, continues to position the United States as a core contributor to global market expansion.
Increasing Regulatory Complexities and Stringent Compliance Requirements to Drive Growth Globally
The expansion of the healthcare regulatory affairs outsourcing market is primarily driven by stricter compliance requirements and increasingly complex regulations. Organizations in the pharmaceutical, biotechnology, and medical device industries are finding it more and more necessary to handle the requirements of safety reporting, approvals, and regulations. These businesses can launch applications faster, run more affordably, and maintain compliance by outsourcing this degree of regulatory expertise. The development of digital tools, trials, and specialized consultants are also driving the market’s adoption and rise.
High Service Costs, Complex Regulations, and Limited Skilled Regulatory Professionals May Hinder Market Expansion Globally
The market for healthcare regulatory affairs outsourcing is largely constrained by high service costs, complicated rules, and a shortage of qualified specialists. Additionally, small pharmaceutical and biotech companies may find it difficult to get specialized regulatory services due to their high cost. Additional operational challenges include a dearth of skilled regulatory experts and a variety of compliance rules. These problems can impede the application of lessons learnt, restrict market expansion, and force businesses to compromise cost-effectiveness for regulatory advantages.
Major Players Analysis Listed in the Healthcare Regulatory Affairs Outsourcing Market Report are
- IQVIA
- PAREXEL International Corporation
- ICON plc
- Charles River Laboratories International, Inc.
- Labcorp / Covance, Inc.
- WuXi AppTec, Inc.
- Medpace, Inc.
- Accell Clinical Research, LLC
- Pharmaceutical Product Development (PPD), LLC
- PRA Health Sciences (now part of ICON)
- Syneos Health
- Genpact Ltd.
- Criterium, Inc.
- ProPharma Group
- Freyr Solutions
- Clinilabs, Inc.
- Promedica International
- Certara, L.P.
- PharmaLex GmbH
- APCER Life Sciences
Need Any Customization Research on Healthcare Regulatory Affairs Outsourcing Market, Enquire Now: https://www.snsinsider.com/enquiry/9049
Segmentation Analysis:
By Service Type
Regulatory Consulting held the largest market share of 28.65% in 2025 on account of extensive advice services assisting pharmaceutical, biotech and medical devices companies in navigating complex regulatory environment effectively. Pharmacovigilance & Safety Reporting is expected to grow at the fastest CAGR of 11.32% during 2026–2033 as a result of market demand for post-market surveillance, adverse event monitoring and patient safety.
By Product Type
Drugs dominated with a 34.12% share in 2025 as conventional small molecule drugs still account for most of the regulatory filings and outsourced compliance requirements. Biologics is projected to expand at the fastest CAGR of 12.15% during the forecast period due to a wide range of complex biologic therapies, vaccines and personal medicine needing specialized regulatory knowledge.
By Stage
Clinical accounted for the highest market share of 42.75% in 2025 as it includes large regulatory paperwork, protocol approvals, and trail management. Post-Market is anticipated to record the fastest CAGR of 11.78% through 2026–2033 due to the increasing regulatory focus on marketed products, drug safety, and real world evidence.
By End-User
Pharmaceutical Companies held the largest share of 38.24% in 2025 due to the Pharmaceutical Companies, which consume the greatest number of regulatory services, including handling submissions, drug registrations and clinical trial approvals. Contract Research Organizations (CROs) are expected to grow at the fastest CAGR of 12.01% during 2026–2033 as it uses outsourced regulatory support to develop their service offerings while handling higher trial volumes.
Regional Insights:
The North America dominated the Healthcare Regulatory Affairs Outsourcing Market with a 41.87% share, owing to the availability of huge pharmaceutical, biopharmaceutical and medical device industry in U.S. and Canada.
The Asia Pacific Healthcare Regulatory Affairs Outsourcing Market is the fastest-growing region, projected to expand at a CAGR of 12.76% during 2026–2033. Growth is a result of the demand for pharmaceutical and biotechnology investment, the expanding number of clinical trials, and stringent regulatory compliance in China, India, Japan, and Australia.
Recent Developments:
- In June 2025, IQVIA launched AI agents for life sciences, enhancing clinical data review, trial planning, and regulatory compliance. The generative IQVIA AI Assistant offers real-time insights, boosting operational efficiency across healthcare projects.
- In October 2025, PAREXEL partnered with Weave Bio to accelerate regulatory submissions using AI-native technology. Additionally, the expanded Site Alliance Program improved site activation speed, patient enrollment, and study start-up efficiency.
Purchase Single User PDF of Healthcare Regulatory Affairs Outsourcing Market Report (20% Discount): https://www.snsinsider.com/checkout/9049
Exclusive Sections of the Report (The USPs):
- REGULATORY SUBMISSION TURNAROUND EFFICIENCY – helps you understand the average turnaround time for outsourced regulatory submissions, enabling benchmarking of speed, responsiveness, and time-to-market advantages.
- OUTSOURCING PENETRATION IN GLOBAL FILINGS – helps you identify the percentage of regulatory filings managed through outsourced partners, indicating industry reliance on external expertise and scalability of regulatory operations.
- DOCUMENT PROCESSING ACCURACY & QUALITY METRICS – helps you assess accuracy rates across outsourced regulatory teams, highlighting quality control strength and risk mitigation in documentation-heavy workflows.
- REGULATORY WORKLOAD VOLUME & SCALE ANALYSIS – helps you evaluate the annual volume of submissions handled by outsourcing firms, reflecting provider capacity, operational maturity, and ability to manage complex portfolios.
- LIFECYCLE STAGE WORKLOAD DISTRIBUTION – helps you understand how outsourced regulatory workloads are distributed across pre-clinical, clinical, and post-market stages, supporting informed outsourcing strategy decisions.
- OPERATIONAL WORKFLOW OPTIMIZATION INDICATORS – helps you gauge overall efficiency of outsourced regulatory operations by integrating turnaround time, volume, and accuracy metrics for performance-driven partner selection.
The Healthcare Regulatory Affairs Outsourcing Market Report delivers comprehensive insights, including:
- Market size and forecasts (2022–2033)
- Detailed segmentation and regional analysis
- Competitive benchmarking and company profiling
- Technology trends, opportunities, and challenges
- Strategic insights for investors and industry stakeholders
Access Complete Report Details of Healthcare Regulatory Affairs Outsourcing Market Analysis & Outlook: https://www.snsinsider.com/reports/healthcare-regulatory-affairs-outsourcing-market-9049
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company’s aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
CONTACT: Contact Us: Rohan Jadhav - Principal Consultant Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK) Email: info@snsinsider.com

Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaShorts takes no editorial responsibility for the same.