The HER2-positive gastric cancer market is set for sustained growth, driven by rising global disease prevalence and expanded HER2 testing that increases the number of patients eligible for targeted therapy. Advances in precision medicine, including the success of trastuzumab and the rapid development of next-generation antibody drug conjugates and combination treatments, are accelerating the adoption of HER2-directed therapies. The wider use of companion diagnostics is strengthening the link between biomarker identification and treatment choice, while expanding indications and multi-line therapy options are further boosting commercial potential. Together, these factors position the market for strong, innovation-driven momentum in the years ahead.
New York, USA, Nov. 27, 2025 (GLOBE NEWSWIRE) — HER2-positive Gastric Cancer Clinical Trial Pipeline: Insights into a Growing Landscape with 20+ Companies Advancing Novel Treatments| DelveInsight
The HER2-positive gastric cancer market is set for sustained growth, driven by rising global disease prevalence and expanded HER2 testing that increases the number of patients eligible for targeted therapy. Advances in precision medicine, including the success of trastuzumab and the rapid development of next-generation antibody drug conjugates and combination treatments, are accelerating the adoption of HER2-directed therapies. The wider use of companion diagnostics is strengthening the link between biomarker identification and treatment choice, while expanding indications and multi-line therapy options are further boosting commercial potential. Together, these factors position the market for strong, innovation-driven momentum in the years ahead.
DelveInsight’s ‘HER2-positive Gastric Cancer Pipeline Insight 2025‘ report provides comprehensive global coverage of pipeline therapies for HER2-positive gastric cancer across various stages of clinical development. The report provides an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance their pipelines and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the HER2-positive gastric cancer domain.
HER2-positive Gastric Cancer Clinical Trial Analysis Summary
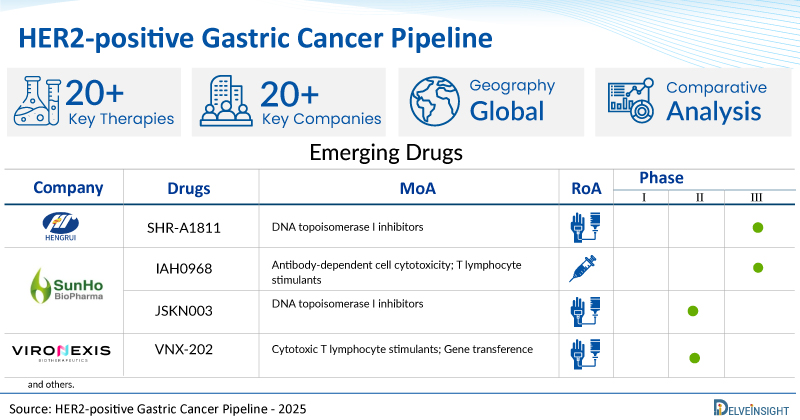
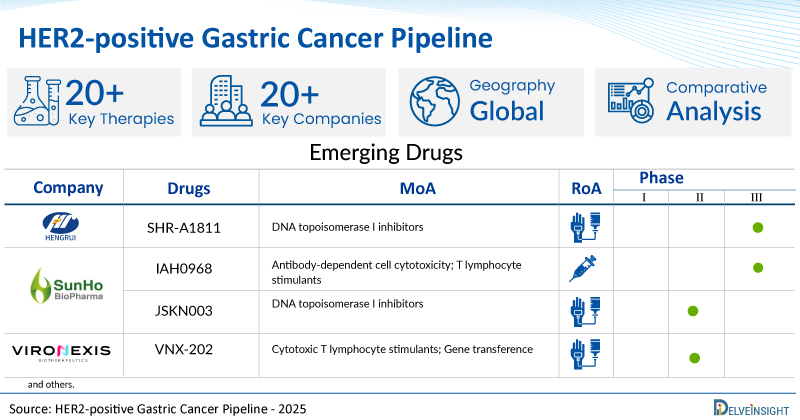
- DelveInsight’s HER2-positive gastric cancer pipeline report depicts a robust space with 20+ active players working to develop 20+ pipeline HER2-positive gastric cancer drugs.
- Key HER2-positive gastric cancer companies such as Jiangsu HengRui Medicine Co., Shanghai Henlius Biotech, AstraZeneca, ALX Oncology Inc., SUNHO(China)BioPharmaceutical CO., Ltd., Shanghai JMT-Bio Inc., Vironexis Biotherapeutics Inc., Enliven Therapeutics, Yuhan Corporation, Mersana Therapeutics, Iambic Therapeutics, Inc., Aurigene Discovery Technologies Limited, SystImmune, Inc., and others are evaluating new HER2-positive gastric cancer drugs to improve the treatment landscape.
- Promising pipeline HER2-positive gastric cancer therapies, such as SHR-A1811, HLX22, Rilvegostomig, Evorpacept, IAH0968, JSKN003, VNX-202, ELVN-002, YH32367, XMT-2056, IAM1363, AUR103, BL-M17D1, and others, are in different phases of HER2-positive gastric cancer clinical trials.
- Approximately 3+ HER2-positive gastric cancer drugs are in the late stage of development, whereas 10+ drugs are in the mid and early stages of development.
- Notable MoAs in HER2-positive gastric cancer clinical trials include DNA topoisomerase I inhibitors, Antibody-dependent cell cytotoxicity; Programmed cell death 1 receptor antagonists; T lymphocyte stimulants; TIGIT protein inhibitors, CD47 antigen inhibitors; Cytotoxic T lymphocyte stimulants; Dendritic cell stimulants; Phagocyte stimulants, Cytotoxic T lymphocyte stimulants; Gene transference, and others.
Request a sample and discover the recent advances in HER2-positive gastric cancer drugs @ HER2-positive Gastric Cancer Pipeline Report
What is HER2-positive Gastric Cancer?
Gastric cancer ranks as the sixth most frequently diagnosed cancer globally and is the third leading cause of cancer-related mortality. In its early stages, it typically presents without symptoms, though some cases are detected incidentally when patients report minor complaints. Most clinical manifestations are associated with advanced disease, with physical signs commonly appearing late. In certain patients, gastric cancer cells overexpress a growth-promoting protein known as HER2. Tumors with elevated HER2 levels are referred to as HER2-positive, and therapies targeting this protein can offer treatment benefits. However, curative outcomes are achieved in only a small proportion of patients undergoing surgical resection, as recurrence is a common occurrence. HER2, also called ErbB2/Neu, is a member of the epidermal growth factor receptor (EGFR) family, located on chromosome 17 (17q21). It encodes a 185 kDa transmembrane glycoprotein (p185). The EGFR family comprises HER1, HER2, HER3, and HER4, each of which contains an extracellular ligand-binding domain, a transmembrane segment, and an intracellular tyrosine kinase domain.
Find out more about HER2-positive Gastric Cancer drugs @ HER2-positive Gastric Cancer Treatment
A snapshot of the Pipeline HER2-positive Gastric Cancer Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| SHR-A1811 | Jiangsu HengRui Medicine Co., Ltd. | III | DNA topoisomerase I inhibitors | Intravenous |
| Rilvegostomig | AstraZeneca | III | Antibody-dependent cell cytotoxicity; Programmed cell death 1 receptor antagonists; T lymphocyte stimulants; TIGIT protein inhibitors | Intravenous |
| Evorpacept | ALX Oncology Inc. | II/III | CD47 antigen inhibitors; Cytotoxic T lymphocyte stimulants; Dendritic cell stimulants; Phagocyte stimulants | Intravenous |
| IAH0968 | SUNHO(China)BioPharmaceutical CO., Ltd. | II/III | Antibody-dependent cell cytotoxicity; T lymphocyte stimulants | Parenteral |
| JSKN003 | SUNHO(China)BioPharmaceutical CO., Ltd. | II | DNA topoisomerase I inhibitors | Intravenous |
| VNX-202 | Vironexis Biotherapeutics Inc. | I/II | Cytotoxic T lymphocyte stimulants; Gene transference | Intravenous |
| ELVN-002 | Enliven Therapeutics | I | Antibody-dependent cell cytotoxicity; Apoptosis stimulants; Tubulin inhibitors | Oral |
Learn more about the emerging HER2-positive gastric cancer therapies @ HER2-positive Gastric Cancer Clinical Trials
Recent Developments in HER2-positive Gastric Cancer Treatment Space
- In November 2025, Jazz Pharmaceuticals plc announced positive top-line results from the Phase III HERIZON-GEA-01 trial evaluating Ziihera® (zanidatamab-hrii) in combination with chemotherapy, with or without the PD-1 inhibitor Tevimbra® (tislelizumab), as first-line treatment for HER2-positive (HER2+) locally advanced or metastatic gastroesophageal adenocarcinoma (GEA), including cancers of the stomach, gastroesophageal junction and esophagus.
- In July 2025, Shanghai Henlius Biotech, Inc. announced that the first patient in the United States has been dosed in the company’s international multicentre phase III head-to-head clinical trial (HLX22-GC-301) comparing its novel anti-HER2 monoclonal antibody (mAb) HLX22 in combination with trastuzumab and chemotherapy with the current first-line standard of care therapy (trastuzumab + chemotherapy ± pembrolizumab) as the first-line treatment for HER2-positive advanced gastric cancer.
- In May 2025, Alphamab Oncology and CSPC Pharmaceutical Group Co., Ltd. jointly announced that a phase II/III clinical trial of anti-HER2 bispecific antibody KN026 in combination with chemotherapy as second-line or above treatment of HER2-positive gastric cancer (GC) (including gastroesophageal junction cancer (GEJ)), has completed the first interim analysis and met the pre-specified primary endpoint of progression-free survival (PFS) as evaluated by an Independent Data Monitoring Committee (IDMC), with both statistical significance and clinical relevance.
- In March 2025, Shanghai Henlius Biotech, Inc. announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) for HLX22, the company’s innovative anti-HER2 monoclonal antibody (mAb) for the treatment of gastric cancer.
- In January 2025, ALX Oncology Holdings Inc., announced positive updated data from the ASPEN-06 Phase II clinical trial demonstrating that the company’s investigational CD47-blocker evorpacept generates a durable clinical response with a well-tolerated safety profile among patients with previously treated HER2-positive advanced gastric cancer (GC) cancer.
- In December 2024, Radiopharm Theranostics announced that it has been granted Belberry Human Research Ethics Committee (HREC) approval in Australia to initiate its First-In-Human (FIH) Phase I therapeutic clinical study of 177Lu-labelled RAD 202 for the treatment of HER2-expressing solid tumors.
- In November 2024, Shanghai Henlius Biotech, Inc. announced that the first patient has been dosed in the phase III international multi-centre clinical trial of HLX22, an innovative anti-HER2 monoclonal antibody (mAb) in combination with trastuzumab and chemotherapy for the first-line treatment of HER2-positive advanced gastric/gastroesophageal junction (G/GEJ) cancer.
- In May 2024, Shanghai Henlius Biotech, Inc. announced that the investigational new drug application (IND) for phase III international multicenter clinical study of Henlius’ novel anti-HER2 mAb, HLX22, in combination with trastuzumab and chemotherapy for the first-line treatment of HER2-positive advanced gastric cancer has been approved by the United States Food and Drug Administration (FDA).
Scope of the HER2-positive Gastric Cancer Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravenous, Subcutaneous, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: DNA topoisomerase I inhibitors, Cytotoxic T lymphocyte stimulants, Immunologic cytotoxicity, Antibody-dependent cell cytotoxicity, Fc gamma receptor IIB antagonists, T lymphocyte stimulants, Immunologic cytotoxicity, Natural killer cell replacements, Tubulin inhibitors
- Key HER2-positive Gastric Cancer Companies: Jiangsu HengRui Medicine Co., Shanghai Henlius Biotech, AstraZeneca, ALX Oncology Inc., SUNHO(China)BioPharmaceutical CO., Ltd., Shanghai JMT-Bio Inc., Vironexis Biotherapeutics Inc., Enliven Therapeutics, Yuhan Corporation, Mersana Therapeutics, Iambic Therapeutics, Inc, Aurigene Discovery Technologies Limited, SystImmune, Inc., and others.
- Key HER2-positive Gastric Cancer Pipeline Therapies: SHR-A1811, HLX22, Rilvegostomig, Evorpacept, IAH0968, JSKN003, VNX-202, ELVN-002, YH32367, XMT-2056, IAM1363, AUR103, BL-M17D1, and others.
Dive deep into rich insights for new HER2-positive Gastric Cancer treatments, visit @ HER2-positive Gastric Cancer Drugs
Table of Contents
| 1. | HER2-positive Gastric Cancer Pipeline Report Introduction |
| 2. | HER2-positive Gastric Cancer Pipeline Report Executive Summary |
| 3. | HER2-positive Gastric Cancer Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | HER2-positive Gastric Cancer Clinical Trial Therapeutics |
| 6. | HER2-positive Gastric Cancer Pipeline: Late-Stage Products (Pre-registration) |
| 7. | HER2-positive Gastric Cancer Pipeline: Late-Stage Products (Phase III) |
| 8. | HER2-positive Gastric Cancer Pipeline: Mid-Stage Products (Phase II) |
| 9. | HER2-positive Gastric Cancer Pipeline: Early-Stage Products (Phase I) |
| 10. | HER2-positive Gastric Cancer Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the HER2-positive Gastric Cancer Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the HER2-positive Gastric Cancer Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the HER2-positive Gastric Cancer pipeline therapeutics, reach out @ HER2-positive Gastric Cancer Therapeutics
Related Reports
HER2+ Gastric Cancer Epidemiology
HER2+ Gastric Cancer Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, and the HER2+ gastric cancer epidemiology trends.
HER2+ Gastric Cancer Market
HER2+ Gastric Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key HER2+ gastric cancer companies including AstraZeneca, Daiichi Sankyo, Merck, Jazz Pharmaceuticals, BeiGene, Zymeworks, Shanghai Henlius Biotech, AbClon, ALX Oncology, Artiva Biotherapeutics, GC Cell, KLUS Pharma, Shanghai Miracogen, Pfizer, Bayer, Enliven Therapeutics, Ambrx, NovoCodex, Mersana Therapeutics, GSK, SystImmune, among others.
Gastric Cancer Market
Gastric Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key gastric cancer companies including Amgen, Jazz Pharmaceuticals, BeiGene, Zymeworks, AstraZeneca, ALX Oncology, Pfizer, KLUS Pharma, Enliven Therapeutics, Ambrx, NovoCodex, among others.
Gastric Cancer Clinical Trial Analysis
Gastric Cancer Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key gastric cancer companies, including EMD Serono, Sumitomo Pharma, LintonPharm Co., Ltd., Rapa Therapeutics LLC, Jiangsu HengRui Medicine Co., Ltd., Janssen Pharmaceutical K.K., Genentech, Exelixis, Bristol-Myers Squibb, Pieris Pharmaceuticals, Inc., Pfizer, Leap Therapeutics, CSPC ZhongQi Pharmaceutical, Ellipses Pharma, Amgen, Hanmi Pharmaceutical, Taiho Oncology, Inc., Shanghai Henlius Biotech, LianBio LLC, Chengdu Kanghong Biotech,Eisai Inc., AB Science, Maxinovel Pharmaceuticals, Shanghai Miracogen Inc., GlaxoSmithKline, Hoffmann-La Roche, Merck Sharp & Dohme, Hutchison Medipharma Limited, Genome & Company, Minneamrita Therapeutics LLC, Suzhou Suncadia Biopharmaceuticals Co., Ltd., MacroGenics, ALX Oncology Inc., Codiak BioSciences, Turning Point Therapeutics, Inc., TCRx Therapeutics, InxMed (Shanghai) Co., Ltd., Imugene Limited, SOTIO Biotech, CARsgen Therapeutics Co., Ltd., Zymeworks Inc., NextCure, Inc., Phanes Therapeutics, Pieris Pharmaceuticals, Inc., Athenex, Inc., Curis, Inc., Qurient Co., Ltd., Acepodia Biotech, Inc., Sichuan Baili Pharmaceutical Co., Ltd., Tarus Therapeutics, Inc., Lumicell, Inc., Legend Biotech,Cue Biopharma, TORL Biotherapeutics, LLC, OBI Pharma, Inc, Astellas Pharma, HiberCell, Inc., Celon Pharma SA, Linnaeus Therapeutics, Inc., Inspirna, Inc., Klus Pharma Inc., Genzada Pharmaceuticals, Shanghai PerHum Therapeutics, VM Oncology, LLC, Immunomic Therapeutics, Peptron, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com

Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaShorts takes no editorial responsibility for the same.