Growing adoption of cardiovascular and urological implants, AI-enabled manufacturing, and demand for cost-efficient care accelerate global market expansion.
Austin, Texas, Jan. 28, 2026 (GLOBE NEWSWIRE) — Nitinol-Based Medical Device Market Size & Growth Analysis
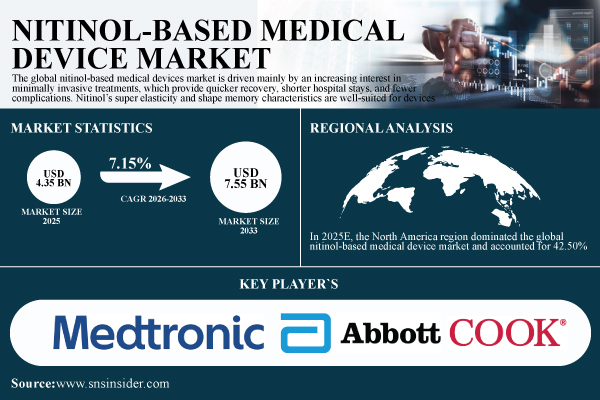
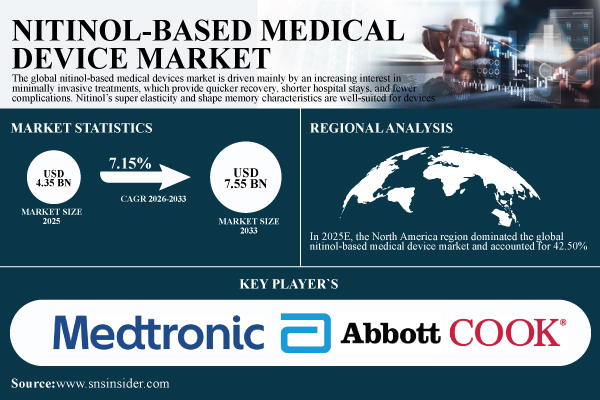
According to SNS Insider, the Nitinol-Based Medical Device Market was valued at USD 4.35 billion in 2025 and is expected to reach USD 7.55 billion by 2033, expanding at a compound annual growth rate (CAGR) of 7.15% during the forecast period 2026–2033. Market growth is primarily driven by the rising preference for minimally invasive medical procedures, which offer reduced recovery time, shorter hospital stays, and lower complication risks.
Market Size and Forecast
- Market Size in 2025: USD 4.35 billion
- Market Size by 2033: USD 7.55 billion
- CAGR: 7.15% from 2026 to 2033
- Base Year: 2025
- Forecast Period: 2026–2033
- Historical Data: 2022–2024
Get a Sample Report of Nitinol-Based Medical Device Market: https://www.snsinsider.com/sample-request/9004
U.S. Nitinol-Based Medical Device Market Outlook
The U.S. Nitinol-Based Medical Device Market was valued at USD 1.58 billion in 2025 and is projected to reach USD 2.70 billion by 2033, growing at a CAGR of 6.97% over 2026–2033.
The United States remains a global trendsetter due to the high prevalence of chronic diseases, affecting nearly 50% of the population, which drives demand for advanced implantable and interventional solutions. Strong physician adoption, rapid clinical validation of innovative devices, and increasing pressure on healthcare systems to improve outcomes while reducing costs continue to support market expansion. Additionally, the widespread use of digital health technologies and data-driven care pathways accelerates innovation and adoption of nitinol-based implants.
Advancements in AI Integration is Propelling Market Expansion Globally
One of the key factors contributing to the growth of the market for nitinol-based medical devices is the integration of AI, which speeds up production, improves quality performance, and allows for product customization. Predictive maintenance, real-time process optimization, and AI-based quality control reduce waste and downtime to provide high precision. These innovations make it possible to produce complicated nitinol implants in a lean, scalable manner. As a result, the market for nitinol-based medical devices is expanding due to more intelligent, quicker, and adaptable manufacture.
Material Variability and Machining Difficulties are Hampering Market Expansion
The primary obstacles to the nitinol medical device market are material variety and challenging machining. Nitinol’s superelasticity and shape memory make it challenging to form the components to tight tolerances and manufacture accurate features, which frequently leads to high reject rates, dispersed dimensions, and surface defects. Furthermore, the production process is complicated by the variety of material qualities. The increase of the nitinol-based medical device market share that would otherwise be required by the worldwide clinical need for high-performance implants is hampered by these difficulties with scalability and regulatory compliance.
Major Players Analysis Listed in the Nitinol-Based Medical Device Market Report are
- Medtronic
- Boston Scientific
- Abbott Laboratories
- Stryker Corporation
- Cardinal Health
- Cook Medical
- C. R. Bard (now part of BD)
- Terumo Corporation
- MicroPort Scientific
- Teleflex Incorporated
- Conformis, Inc.
- Merit Medical Systems, Inc.
- Endologix, Inc.
- B. Braun Melsungen AG
- Gore & Associates
- BD (Becton, Dickinson and Company)
- Olympus Corporation
- A. Bellucci (or similar niche Nitinol device innovators)
- Cochlear Limited
- Olympus Terumo Biomaterials (OTB)
Need Any Customization Research on Nitinol-Based Medical Device Market, Enquire Now: https://www.snsinsider.com/enquiry/9004
Segmentation Analysis:
By Product Type
Retrival device was the dominant in the nitinol-based medical device market analysis, with a 38.60% market share in 2025, owing to their important role in the less-invasive image-guided treatments, including neuro-vascular and cardio-vascular interventions. Catheters are emerging as the fastest-growing segment in the nitinol-based medical device market trend, with a CAGR of 7.71% in the forecasted period 2026-2033, due to their growing application in urology, cardiology, and neurovascular procedures.
By Application
In 2025, the cardiovascular, controlled nitinol-based medical device market had with 62.64% market share, owing to the high proportion of patients globally who have ischemic heart disease and peripheral artery disease. The Others segment, including orthopedic staples, neurovascular clot retrievers, and gastrointestinal stents, is the fastest-growing segment in the global nitinol-based medical device market trend, owing to broadening uses in areas other than cardiology and urology.
By End-User
In 2025, the Hospital controls the global nitinol-based medical device industry with a significant market share of 65.80% owing to their high productivity in performing complex procedures, including cardiovascular and neurovascular interventions. In the global nitinol-based medical device industry, the ambulatory surgical Centers segment plays a vital role, registering the fastest growth over the forecast period, fueled by the increasing tendency to perform outpatient operations.
Regional Insights:
In 2025E, the North America region dominated the global nitinol-based medical device market and accounted for 42.50% of the overall revenue share, owing to its developed medical infrastructure, high incidence of chronic diseases, and the presence of established medical equipment companies.
The Asia Pacific region is projected to grow with the fastest CAGR of 7.84% over the forecast period 2026-2033, owing to the growing advent of healthcare infrastructures, increased awareness about minimally invasive procedures, and high incidence of chronic disorders, including cardiovascular and urological diseases.
Recent Developments:
- In 2025, Medtronic expanded its nitinol-enabled endovascular platform with enhanced delivery system upgrades designed to improve navigability and precision for complex peripheral interventions.
- In 2025, Boston Scientific introduced a next-generation nitinol peripheral stent platform featuring improved radial strength and enhanced durability for long-segment arterial disease treatment.
Purchase Single User PDF of Nitinol-Based Medical Device Market Report (20% Discount): https://www.snsinsider.com/checkout/9004
Exclusive Sections of the Report (The USPs):
- MATERIAL PERFORMANCE & FATIGUE RELIABILITY METRICS – helps you evaluate fatigue resistance cycles, elasticity recovery rates, and shape-memory activation ranges of Nitinol across medical device applications.
- CLINICAL ADOPTION & PROCEDURE VOLUME ANALYSIS – helps you understand global procedure volumes using Nitinol-based implants and penetration levels versus stainless steel and cobalt-chrome alternatives.
- DEVICE SAFETY & FAILURE RATE BENCHMARKS – helps you assess revision and failure rates of Nitinol devices compared with other biomaterials to support material selection decisions.
- MANUFACTURING YIELD & PROCESS EFFICIENCY METRICS – helps you measure yield improvements, precision machining adoption, and lead-time reductions achieved through advanced Nitinol processing technologies.
- ADVANCED MANUFACTURING ADOPTION RATE – helps you uncover opportunities linked to additive manufacturing, laser cutting, and automation trends in Nitinol component production.
- REGULATORY APPROVAL & QUALITY COMPLIANCE TRACKING – helps you gauge FDA and CE approval trends, biocompatibility and corrosion testing requirements, and recall frequency linked to Nitinol performance.
About the Report
The Nitinol-Based Medical Device Market Report delivers comprehensive insights, including:
- Market size and forecasts (2022–2033)
- Detailed segmentation and regional analysis
- Competitive benchmarking and company profiling
- Technology trends, opportunities, and challenges
- Strategic insights for investors and industry stakeholders
Access Complete Report Details of Nitinol-Based Medical Device Market Analysis & Outlook: https://www.snsinsider.com/reports/nitinol-based-medical-device-market-9004
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company’s aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
CONTACT: Contact Us: Rohan Jadhav - Principal Consultant Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK) Email: info@snsinsider.com

Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaShorts takes no editorial responsibility for the same.