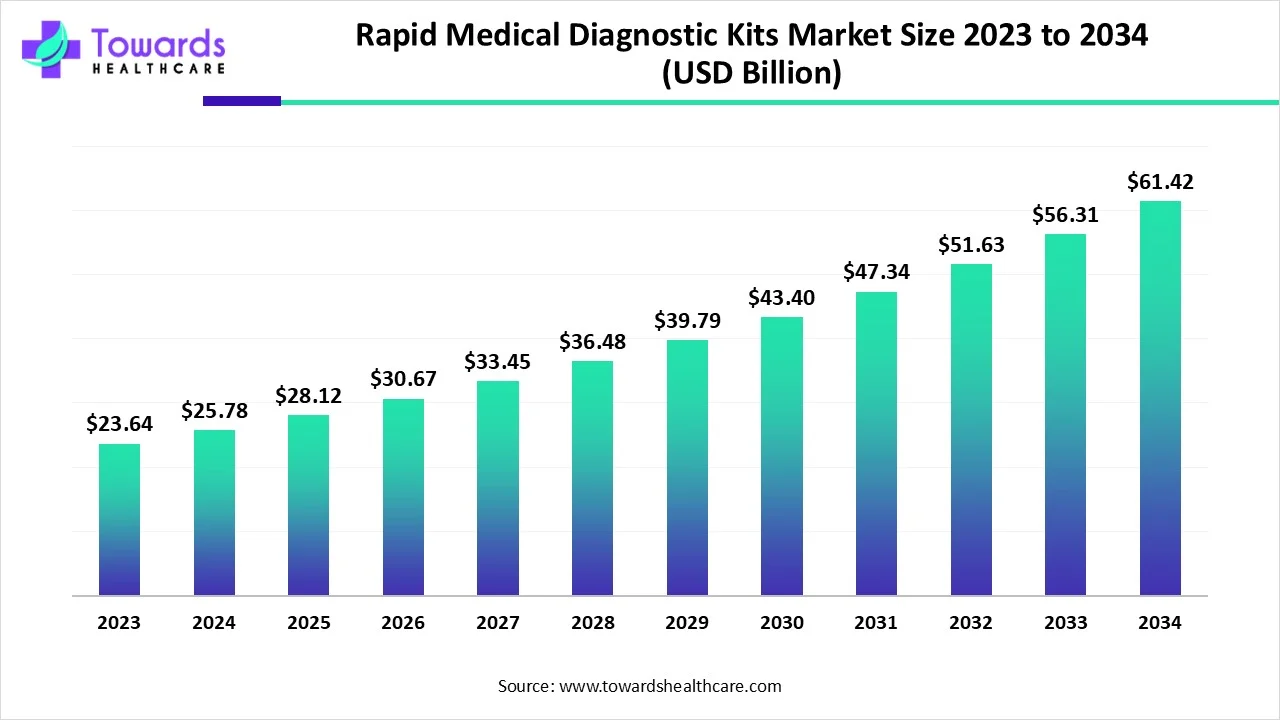
Rapid Medical Diagnostic Kits Market to Surpass USD 61.42 Billion by 2034, Driven by Expanding Healthcare Applications and Rising Health Awareness
The global rapid medical diagnostic kits market size is calculated at USD 28.12 billion in 2025 and is expected to reach around USD 61.42 billion by 2034, growing at a CAGR of 9.07% for the forecasted period.
Ottawa, Nov. 03, 2025 (GLOBE NEWSWIRE) — The global rapid medical diagnostic kits market size was valued at USD 25.78 billion in 2024 and is predicted to hit around USD 61.42 billion by 2034, rising at a 9.07% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research. The global rapid medical diagnostic kits market is driven by the expanding healthcare applications and growing health awareness.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Key Takeaways
- North America held a major revenue of 37% share of the market in 2024.
- Asia Pacific is expected to witness the fastest growth in the rapid medical diagnostic kits market during the forecast period.
- By product type, the over-the-counter (OTC) kits segment led the market in 2024 and is expected to witness the fastest growth during the forecast period.
- By technology type, the lateral flow segment led the market in 2024 and is expected to witness the fastest growth during the forecast period.
- By application type, the blood glucose testing segment led the global market in 2024 and is expected to witness the fastest growth during the forecast period.
- By end-user type, the hospitals segment led the market in 2024.
- By end-user type, the home care segment is expected to witness significant growth during the forecast period.
What are the Rapid Medical Diagnostic Kits?
The rapid medical diagnostic kits market is driven by heightened health awareness, technological advancements, and incidences of chronic and infectious diseases. The rapid medical diagnostic kits refer to the compact, fast, reliable, and portable test systems used to diagnose conditions immediately without the use of complex laboratory equipment. These kits are used for early detection of diseases, point-of-care testing (POCT), home-based health monitoring, pregnancy or fertility testing, monitoring treatment effectiveness, chronic disease management, and drug and alcohol testing.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5522
What are the Major Growth Drivers in the Market?
The increasing demand for point-of-care testing is the major driver in the rapid medical diagnostic kits market, which is driven by increasing demand for rapid results. Moreover, their easy-to-use application is increasing their use at home testing and chronic disease management, which decreases the patients’ dependence on hospitals or labs, enhancing their convenience and comfort. Moreover, increasing disease prevalence, technological innovations, and a shift towards decentralized healthcare are other market drivers.
What are the Key Drifts in the Market?
The rapid medical diagnostic kits market has been expanding due to the growing funding and collaborations to launch and enhance the use of various rapid diagnostic kits.
- In October 2025, to advance the non-invasive fertility test, a total of $1.2 million was secured by Genie Fertility. With an aim to identify reproductive health, this test will use a machine learning platform to detect molecular markers in the menstrual blood.
- In March 2025, a total of $1 million in pre-seed funding was secured by Arva Health, which will be utilized to support its mission to provide more affordable, accessible, and stigma-free reproductive healthcare in India and will scale its tech-enabled fertility clinics.
- In January 2025, a total of $3 million in funding was secured by Gravidas Diagnostics, Inc., which is a pioneering force in maternal health. Moreover, to enhance the development of its rapid point-of-care (PoC) test to identify the risk of severe, life-threatening preeclampsia in pregnant women will be supported by this funding.
- In January 2025, a research program was conducted by a collaboration between the University of Auckland, the University of Otago, local research partners, health providers, and communities to develop a tool predicting the risk and mitigating the effects of flu and other respiratory illnesses, which was awarded $8.3 million in a grant.
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
What is the Significant Challenge in the Market?
Accuracy concerns are the major challenge in the rapid medical diagnostic kits market. These kits may provide false positive or false negative results, which may lead to unnecessary treatments or no treatment for the patients, reducing their use. Additionally, other market challenges include the limited shelf life of the kits, storage issues, and competition from traditional laboratory tests.
Regional Analysis
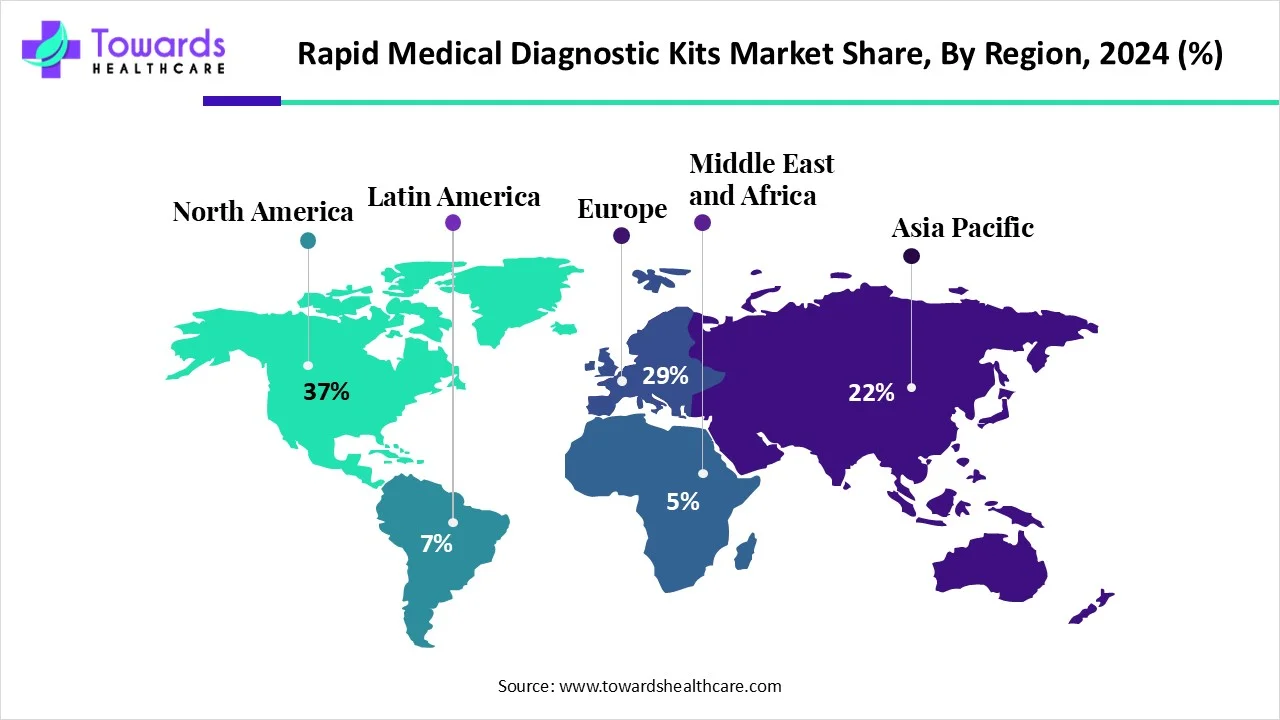
How did North America Dominate the Rapid Medical Diagnostic Kits Market in 2024?
In 2024, North America captured the biggest revenue share of 37% in the rapid medical diagnostic kits market, due to a growth in the incidence rate of infectious diseases, diabetes, and increasing public awareness, which increased the use of rapid diagnostic kits, driving home care. The presence of advanced healthcare systems has increased their use in daily practices, where the industries are focused on developing advanced kits with enhanced accuracy, speed, and digital connectivity. Thus, these advancements contributed to the market growth.
Download the single region market report @ https://www.towardshealthcare.com/checkout/5522
Asia Pacific Rapid Medical Diagnostic Kits Market Trends:
Asia Pacific is expected to host the fastest growth in the rapid medical diagnostic kits market during the forecast period, due to the presence of a large population base, which increases the risk and incidence of chronic and infectious diseases, which promotes the use of rapid diagnostic kits. The growing accessibility and affordability are increasing the self-testing trends, increasing their use. Their demand is also increasing due to growing government screening programs, where all these advancements are enhancing the market growth.
Segmental Insights
How Did the Over-The-Counter (OTC) Kits Segment Dominate in the Market in 2024?
By product type, the over-the-counter (OTC) kits segment held the largest share of the rapid medical diagnostic kits market in 2024 and is expected to show the highest growth during the predicted time, due to their enhanced accessibility. At the same time, the growing shift towards self-testing has increased its adoption, which has enhanced patient comfort as well. Moreover, their affordability also contributed to their increased use.
Which Technology Type Segment Held the Dominating Share of the Market in 2024?
By technology type, the lateral flow segment held the dominating share of the rapid medical diagnostic kits market in 2024 and is expected to show the fastest growth rate during the predicted time, driven by its rapid results. The kits developed using this technology were easy to use, affordable, and portable. Moreover, they were widely used for various applications.
What Made Blood Glucose Testing the Dominant Segment in the Market in 2024?
By application type, the blood glucose testing segment held the largest share of the global rapid medical diagnostic kits market in 2024 and is expected to show the highest growth during the upcoming years, due to a growth in the incidence of diabetes and its awareness. This, in turn, increased the demand for blood glucose testing. Additionally, self-testing and routine monitoring also contributed to the increased use of rapid diagnostic kits.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
How the Hospitals Segment Dominated the Market in 2024?
By end-user type, the hospitals segment held the dominating share of the rapid medical diagnostic kits market in 2024, driven by a high volume of patients. At the same time, a wide range of diseases or conditions also increased the use of rapid medical diagnostic kits. Furthermore, growth in the screening programs also increased their use.
By end-user type, the home care segment is expected to show lucrative growth during the upcoming years. The growing geriatric population and self-testing trends are increasing the use of rapid medical diagnostics kits at home. Moreover, they are being used for chronic disease management, which is improving patient convenience and comfort.
Recent Developments in the Market
- In July 2025, LensHooke X12 PRO, an advanced AI-powered semen analyzer, was launched on World IVF Day by Ferty9 Fertility Center.
- In May 2025, for swift respiratory virus detection, an All-in-One SARS-CoV-2, Flu A/B & RSV Antigen Rapid Test Kit was launched by BABIO, which provides results within 15 mins.
- In February 2025, a qPCR test for the detection of H5 avian influenza A variants, a rapid and accurate detection test that is Rapid H5 was launched by MicroGenDX.
Browse More Insights of Towards Healthcare:
The U.S. oncology molecular diagnostics market was valued at USD 810 million in 2024, growing to USD 909.14 million in 2025, and is projected to reach approximately USD 2,570.17 million by 2034, expanding at a CAGR of 12.24% from 2025 to 2034.
Globally, the oncology molecular diagnostics market was valued at USD 3.11 billion in 2024, rising to USD 3.48 billion in 2025, and is expected to reach around USD 9.76 billion by 2034, registering a CAGR of 12.13% during the forecast period.
The global cervical cancer diagnostics market stood at USD 5.08 billion in 2024, increased to USD 5.37 billion in 2025, and is anticipated to reach nearly USD 8.87 billion by 2034, growing at a CAGR of 5.74% from 2024 to 2034.
The exosome diagnostics and therapeutics market was valued at USD 33.04 million in 2023 and is projected to surge to USD 22,609.77 million by 2034, witnessing an exceptional CAGR of 81.03% between 2024 and 2034.
The DNA diagnostics market was estimated at USD 10.69 billion in 2023 and is expected to reach USD 17.44 billion by 2034, expanding at a CAGR of 4.55% during 2024–2034.
The global immunodiagnostics market was valued at USD 19.1 billion in 2023 and is predicted to grow substantially to USD 70.9 billion by 2034, progressing at a CAGR of 12.84% from 2024 to 2034.
The companion diagnostic technologies market was valued at USD 2.78 billion in 2023 and is forecasted to reach USD 9.06 billion by 2034, advancing at a CAGR of 11.34% during the same period.
The core clinical molecular diagnostics market stood at USD 5.3 billion in 2023 and is projected to grow to USD 14.45 billion by 2034, registering a CAGR of 9.54% from 2024 to 2034.
The AI in diagnostics market was valued at USD 1.12 billion in 2023 and is expected to reach USD 12.65 billion by 2034, expanding at a robust CAGR of 24.64% over the forecast timeline.
The global in vitro diagnostics market was valued at USD 77.95 billion in 2023 and is estimated to reach USD 123.45 billion by 2034, growing at a CAGR of 4.45% between 2024 and 2034.
Rapid Medical Diagnostic Kits Market Key Players List

- Danaher Corporation
- Abbott
- Alfa Scientific Designs, Inc.
- F. Hoffmann-La Roche AG
- Meridian Bioscience, Inc.
- Artron Laboratories Inc.
- BTNX, Inc.
- bioMerieux SA
- Cardinal Health
- Trinity Biotech
- BD
- Creative Diagnostics
- ACON Laboratories, Inc.
- McKesson Medical-Surgical, Inc.
- Bio-Rad Laboratories, Inc.
- Sight Diagnostics Ltd.
- Zoetis
Segments Covered in The Report
By Product
- Over-the-counter (OTC) Kits
- Professional Kits
By Technology
- Lateral Flow
- Agglutination
- Solid Phase
- Other Technologies
By Application
- Blood Glucose Testing
- Infectious Disease Testing
- COVID-19
- Hepatitis
- HIV
- Influenza
- Others
- Cardiometabolic Testing
- Pregnancy and Fertility Testing
- Fecal Occult Blood Testing
- Coagulation Testing
- Toxicology Testing
- Lipid Profile Testing
- Other Applications
By End-user
- Hospitals & Clinics
- Home Care
- Diagnostic Laboratories
By Region
North America
- U.S.
- Canada
- Mexico
- Rest of North America
South America
- Brazil
- Argentina
- Rest of South America
Europe
- Western Europe
- Germany
- Italy
- France
- Netherlands
- Spain
- Portugal
- Belgium
- Ireland
- UK
- Iceland
- Switzerland
- Poland
- Rest of Western Europe
- Eastern Europe
- Austria
- Russia & Belarus
- Türkiye
- Albania
- Rest of Eastern Europe
Asia Pacific
- China
- Taiwan
- India
- Japan
- Australia and New Zealand,
- ASEAN Countries (Singapore, Malaysia)
- South Korea
- Rest of APAC
MEA
- GCC Countries
- Saudi Arabia
- United Arab Emirates (UAE)
- Qatar
- Kuwait
- Oman
- Bahrain
- South Africa
- Egypt
- Rest of MEA
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/5522
Access our exclusive, data-rich dashboard dedicated to the healthcare market – built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaShorts takes no editorial responsibility for the same.
